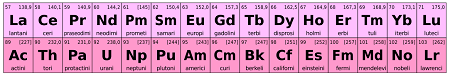
The f-block elements are a group of elements in the periodic table that are also known as inner transition elements. They are located in the two rows located below the main body of the periodic table, separated by the transition metals.
The f-block elements are divided into two groups: the lanthanides and the actinides. The lanthanides include the elements from atomic number 57 (lanthanum) to 71 (lutetium), while the actinides include the elements from atomic number 89 (actinium) to 103 (lawrencium).
Some important characteristics of f-block elements include:
- They have partially filled f-orbitals, which give them unique electronic and magnetic properties.
- They tend to have high melting and boiling points.
- They tend to form complex ions and compounds.
- They exhibit a wide range of oxidation states.
Some specific topics related to f-block elements that are important for JEE (Main+Advanced) include:
- Electronic configuration of f-block elements
- Lanthanide contraction
- Magnetic properties of f-block elements
- Extraction of actinides
- Complex formation of f-block elements
It’s important to have a good understanding of the properties and trends of f-block elements to be successful in JEE (Main+Advanced) chemistry.
What is Required JEE (Main+Advance) e-Intermediate Course f-Block Elements
To master the topic of f-block elements for JEE (Main+Advanced), it is important to cover the following areas:
- Electronic Configuration of F-Block Elements: It is essential to understand the electronic configuration of the f-block elements, which involves the filling of the f-orbitals. This helps to explain the unique properties of these elements.
- Lanthanide Contraction: This refers to the decrease in atomic and ionic radii of the elements as you move from left to right in the lanthanide series. Understanding this phenomenon is important for predicting properties of the elements.
- Magnetic Properties of F-Block Elements: F-block elements exhibit unique magnetic properties due to their partially filled f-orbitals. It is essential to understand these properties to predict their behavior in magnetic fields.
- Extraction of Actinides: Actinides are radioactive and have unique chemical properties. Understanding the extraction and purification methods for these elements is important in their industrial applications.
- Complex Formation of F-Block Elements: F-block elements tend to form complex ions and compounds. It is important to understand the nature of these complexes and their properties.
To master these concepts, it is important to study from a comprehensive textbook, take notes, and solve practice problems. Additionally, online resources such as video lectures, online quizzes, and forums can be very helpful in reinforcing your understanding of the material. Practicing with JEE-level questions and past papers is also important to prepare for the exam.
When is Required JEE (Main+Advance) e-Intermediate Course f-Block Elements
The topic of f-block elements is an important part of the JEE (Main+Advanced) chemistry syllabus, and is usually covered in the 11th and 12th grade. In the JEE (Main+Advanced) examination, questions related to f-block elements may appear in both the Paper 1 and Paper 2 exams.
It is recommended to study f-block elements thoroughly in the 11th and 12th grade, and to revise the topic in the months leading up to the JEE (Main+Advanced) examination. The JEE (Main+Advanced) examination is usually held in the months of April and May, so it is important to have a solid understanding of f-block elements well before this time.
Additionally, students may choose to enroll in JEE (Main+Advanced) e-Intermediate courses or coaching classes to supplement their studies and to receive expert guidance and support in preparing for the exam. These courses may cover f-block elements and other important topics in depth, and may also include mock tests and practice papers to help students prepare for the exam.
Where is Required JEE (Main+Advance) e-Intermediate Course f-Block Elements
JEE (Main+Advanced) e-Intermediate courses for f-block elements are available online and can be accessed from anywhere with an internet connection. Many coaching institutes offer online courses for JEE (Main+Advanced) preparation, which cover all the topics in the syllabus, including f-block elements.
Students can enroll in these courses and access video lectures, study materials, online quizzes and tests, and other resources from the comfort of their homes. These courses may also include doubt-solving sessions, mentorship, and guidance from experienced JEE trainers.
There are many online platforms and websites that offer JEE (Main+Advanced) e-Intermediate courses for f-block elements, including Vedantu, BYJU’s, Unacademy, Toppr, and many others. Students can choose a course that suits their learning style and preferences and enroll in it to supplement their preparation for the exam.
How is Required JEE (Main+Advance) e-Intermediate Course f-Block Elements
JEE (Main+Advanced) e-Intermediate courses for f-block elements are designed to provide students with a comprehensive understanding of the topic and help them prepare for the exam. These courses may include the following features:
- Video Lectures: The courses may include video lectures that cover all the important concepts related to f-block elements in a detailed and engaging manner. The lectures may be delivered by experienced JEE trainers who have a deep understanding of the subject.
- Study Materials: The courses may provide study materials, including notes, textbooks, and other resources, that are designed to help students reinforce their understanding of the topic.
- Online Quizzes and Tests: The courses may include online quizzes and tests that are designed to help students assess their understanding of the topic and identify areas where they need to improve. These quizzes and tests may include JEE-level questions and may provide detailed feedback to help students improve their performance.
- Doubt-Solving Sessions: The courses may include doubt-solving sessions where students can ask questions and get their doubts clarified by experienced trainers. These sessions may be conducted online and may provide students with individual attention and support.
- Mentorship and Guidance: The courses may provide mentorship and guidance to students, helping them stay motivated and focused on their preparation for the exam.
Overall, JEE (Main+Advanced) e-Intermediate courses for f-block elements are designed to help students master the topic and perform well in the exam. These courses may provide students with the guidance, support, and resources they need to achieve their goals and succeed in the exam.
Nomenclature of JEE (Main+Advance) e-Intermediate Course f-Block Elements
Nomenclature of f-block elements is an important part of the JEE (Main+Advanced) chemistry syllabus. Here are some important nomenclature rules for f-block elements:
- Lanthanide Series: The 14 elements with atomic numbers ranging from 58 to 71 are known as the lanthanide series. They are named after the element with the atomic number of 57, which is lanthanum. The rest of the elements in the series are named in order of their atomic numbers, with the suffix “-ium” added to the stem of the element name. For example, the element with atomic number 59 is named praseodymium.
- Actinide Series: The 14 elements with atomic numbers ranging from 90 to 103 are known as the actinide series. They are named after the element with the atomic number of 89, which is actinium. The rest of the elements in the series are named in order of their atomic numbers, with the suffix “-ium” added to the stem of the element name. For example, the element with atomic number 95 is named americium.
- Complex Ions: Complex ions are named by listing the ligands first, in alphabetical order, followed by the central metal ion. For example, the complex ion [Co(NH3)6]3+ is named hexaamminecobalt(III) ion.
- Coordination Compounds: Coordination compounds are named by listing the ligands first, in alphabetical order, followed by the name of the central metal ion, with a Roman numeral indicating the oxidation state of the metal ion. For example, the compound [Fe(CN)6]4- is named potassium hexacyanoferrate(II).
- Oxides and Hydroxides: Oxides and hydroxides of f-block elements are named by using prefixes such as “di-“, “tri-“, “tetra-“, etc. to indicate the number of atoms of oxygen or hydroxide in the compound. For example, the compound CeO2 is named cerium(IV) oxide.
It is important to study and practice nomenclature of f-block elements to ensure that you can recognize and name compounds correctly on the exam.
Case Study on JEE (Main+Advance) e-Intermediate Course f-Block Elements
One example of a case study on the JEE (Main+Advanced) e-Intermediate course for f-block elements is as follows:
Case Study: Ashok is a JEE aspirant who is struggling with the chemistry section of the exam. He finds the topic of f-block elements particularly challenging and is unsure about how to approach it. He decides to enroll in a JEE (Main+Advanced) e-Intermediate course for f-block elements to supplement his preparation for the exam.
Solution: Ashok enrolls in an online JEE (Main+Advanced) e-Intermediate course for f-block elements offered by a reputed coaching institute. The course covers all the important concepts related to f-block elements and provides Ashok with access to a range of resources to help him master the topic. Here are some key features of the course that help Ashok improve his performance:
- Video Lectures: The course includes video lectures that are designed to help Ashok understand the concepts related to f-block elements in a detailed and engaging manner. The lectures are delivered by experienced JEE trainers who have a deep understanding of the subject.
- Study Materials: The course provides study materials, including notes, textbooks, and other resources, that are designed to help Ashok reinforce his understanding of the topic. The study materials are comprehensive and cover all the important concepts related to f-block elements.
- Online Quizzes and Tests: The course includes online quizzes and tests that are designed to help Ashok assess his understanding of the topic and identify areas where he needs to improve. The quizzes and tests include JEE-level questions and provide detailed feedback to help Ashok improve his performance.
- Doubt-Solving Sessions: The course includes doubt-solving sessions where Ashok can ask questions and get his doubts clarified by experienced trainers. These sessions are conducted online and provide Ashok with individual attention and support.
- Mentorship and Guidance: The course provides mentorship and guidance to Ashok, helping him stay motivated and focused on his preparation for the exam. The trainers provide Ashok with personalized feedback and guidance on how to improve his performance in the chemistry section of the exam.
As a result of enrolling in the JEE (Main+Advanced) e-Intermediate course for f-block elements, Ashok is able to improve his performance in the chemistry section of the exam. He gains a comprehensive understanding of the topic and is able to recognize and name compounds correctly on the exam. With the guidance and support provided by the course, Ashok is able to achieve his goals and perform well in the exam.
White paper on JEE (Main+Advance) e-Intermediate Course f-Block Elements
Introduction:
The Joint Entrance Examination (JEE) is a highly competitive engineering entrance exam in India that is conducted for admission into various undergraduate engineering programs offered by the Indian Institutes of Technology (IITs), National Institutes of Technology (NITs), and other prestigious engineering colleges across the country. The JEE (Main+Advanced) exam tests the aspirants’ knowledge of physics, chemistry, and mathematics. In this white paper, we will focus on the f-block elements and discuss the importance of an e-Intermediate course for JEE (Main+Advanced) aspirants to master this topic.
Importance of f-Block Elements in JEE (Main+Advanced) Exam:
The f-block elements consist of the lanthanides and actinides, which occupy the two rows at the bottom of the periodic table. These elements have unique electronic configurations and exhibit a range of interesting properties. As a result, f-block elements are an important topic in JEE (Main+Advanced) chemistry syllabus. Questions related to f-block elements can be asked in both JEE Main and JEE Advanced exams.
The JEE (Main+Advanced) exam tests the aspirants’ understanding of the concepts related to f-block elements, including their electronic configuration, properties, and their reactions with different ligands. Aspirants are also expected to know the nomenclature and structures of complex compounds of f-block elements. To perform well in the chemistry section of the exam, it is essential for aspirants to have a clear understanding of the f-block elements.
Why an e-Intermediate Course is Essential for F-Block Elements:
Many JEE (Main+Advanced) aspirants find the f-block elements a challenging topic to master. The topic requires a deep understanding of electronic configurations, ligands, and complex compounds. Aspirants need to have a strong conceptual foundation and should be able to apply the knowledge in solving problems. This is where an e-Intermediate course for f-block elements can be helpful.
An e-Intermediate course is an online course that is designed to supplement the preparation of JEE (Main+Advanced) aspirants. The course is delivered online, which allows aspirants to learn at their own pace and convenience. The course covers all the important concepts related to f-block elements and provides aspirants with access to a range of resources to help them master the topic.
The course includes video lectures, study materials, online quizzes and tests, doubt-solving sessions, and mentorship and guidance. The video lectures are delivered by experienced JEE trainers who have a deep understanding of the subject. The study materials are comprehensive and cover all the important concepts related to f-block elements. The online quizzes and tests are designed to help aspirants assess their understanding of the topic and identify areas where they need to improve. The doubt-solving sessions provide aspirants with individual attention and support. The mentorship and guidance help aspirants stay motivated and focused on their preparation for the exam.
Conclusion:
In conclusion, the f-block elements are an important topic in the JEE (Main+Advanced) chemistry syllabus. To perform well in the chemistry section of the exam, it is essential for aspirants to have a clear understanding of the f-block elements. An e-Intermediate course for f-block elements can be helpful in providing aspirants with a deep understanding of the topic and help them master the concepts related to f-block elements. With the right guidance and support, aspirants can achieve their goals and perform well in the JEE (Main+Advanced) exam.
