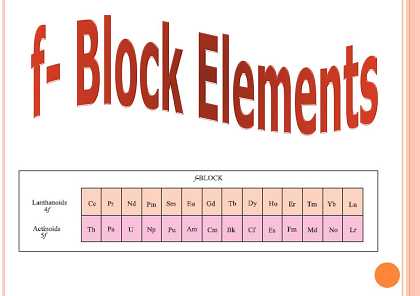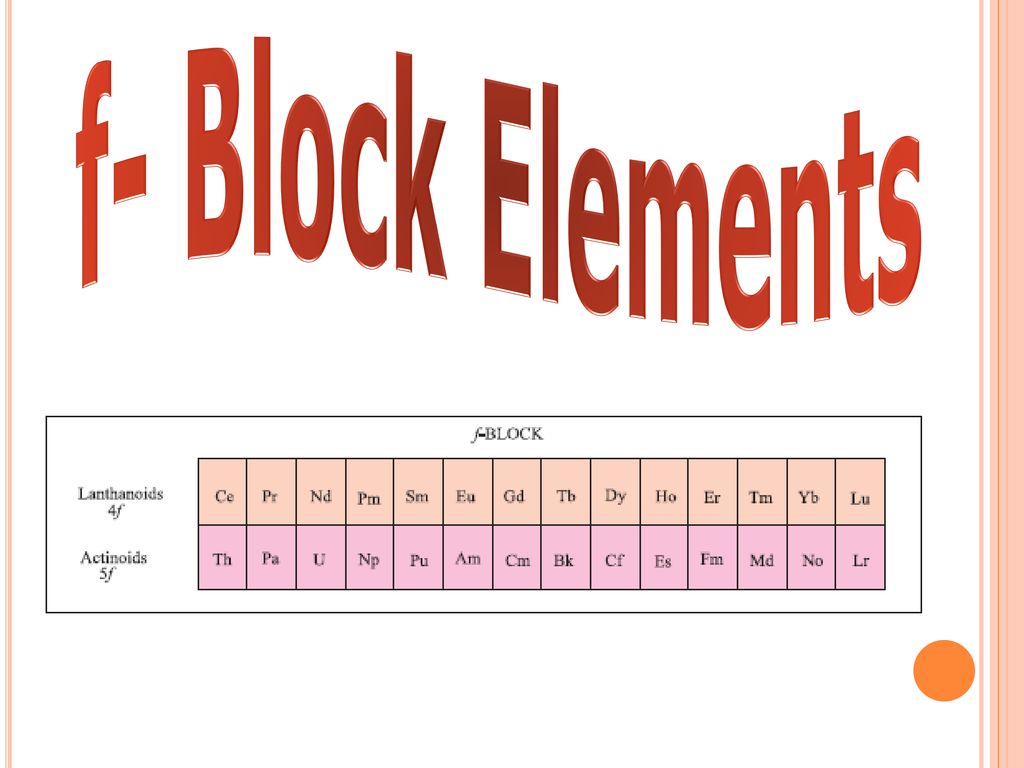
The f-block elements, also known as inner transition metals, are a group of elements located in the f-block of the periodic table. These elements include the lanthanides and actinides, which have partially filled f-orbitals.
Here are some important topics that you should cover in your JEE (Main+Advance) Intermediate Course for f-Block Elements:
- Electronic configuration: The electronic configuration of f-block elements is quite complex due to the presence of partially filled f-orbitals. You should be familiar with the Aufbau principle and Hund’s rule, which are used to determine the electronic configuration of these elements.
- Oxidation states: The f-block elements exhibit a wide range of oxidation states, which can be attributed to the presence of partially filled f-orbitals. You should be familiar with the common oxidation states of these elements and the factors that influence them.
- Lanthanides: The lanthanides are a group of 15 elements that have similar properties. You should be familiar with their physical and chemical properties, including their atomic and ionic radii, melting and boiling points, and reactivity.
- Actinides: The actinides are a group of 15 elements that are radioactive and have a wide range of applications. You should be familiar with their physical and chemical properties, including their atomic and ionic radii, melting and boiling points, and reactivity.
- Complex formation: The f-block elements are known for their ability to form complex ions due to the presence of partially filled f-orbitals. You should be familiar with the different types of ligands and their effect on the stability of complex ions.
- Magnetic properties: The f-block elements exhibit a wide range of magnetic properties due to the presence of partially filled f-orbitals. You should be familiar with the different types of magnetism, such as paramagnetism and diamagnetism, and their relationship to the electronic configuration of these elements.
- Applications: The f-block elements have a wide range of applications, including in nuclear reactors, catalysis, and lighting. You should be familiar with some of the important applications of these elements.
In summary, the f-block elements are an important group of elements that have unique physical and chemical properties due to the presence of partially filled f-orbitals. You should be familiar with their electronic configuration, oxidation states, physical and chemical properties, complex formation, magnetic properties, and applications.
History of JEE (Main+Advance) Intermediate Course f-Block Elements
The study of f-block elements has been an important part of chemistry for many decades. The history of the JEE (Main+Advance) Intermediate Course for f-Block Elements can be traced back to the early 20th century, when chemists began to investigate the properties of these elements in more detail.
In 1913, the Danish physicist Niels Bohr proposed his atomic model, which explained the electronic structure of atoms. This model helped to explain the complex electronic configuration of f-block elements, which have partially filled f-orbitals. In the years that followed, chemists began to investigate the properties of these elements in more detail.
In the 1920s and 1930s, chemists began to isolate and study individual f-block elements. For example, in 1929, the American chemist Edwin McMillan isolated neptunium, the first synthetic element beyond uranium. In 1944, Glenn Seaborg and his team at the University of California, Berkeley, discovered plutonium, which became an important element in the development of nuclear weapons.
In the decades that followed, chemists continued to study the properties of f-block elements and their applications. In the 1970s and 1980s, for example, lanthanide compounds were developed as catalysts for various chemical reactions. In the 1990s and 2000s, actinide elements were studied for their potential applications in nuclear reactors and waste storage.
Today, the JEE (Main+Advance) Intermediate Course for f-Block Elements covers a wide range of topics related to the electronic structure, physical and chemical properties, complex formation, magnetic properties, and applications of these elements. Students learn about the historical context of the study of f-block elements, as well as the latest research and developments in this field.
Nature of JEE (Main+Advance) Intermediate Course f-Block Elements
The JEE (Main+Advance) Intermediate Course for f-Block Elements is a rigorous and comprehensive course that covers the properties and applications of the elements in the f-block of the periodic table. This course is designed to provide students with a deep understanding of the electronic structure, physical and chemical properties, complex formation, magnetic properties, and applications of these elements.
The nature of the JEE (Main+Advance) Intermediate Course for f-Block Elements is both theoretical and practical. Students learn about the theoretical aspects of the subject, such as the electronic configuration and oxidation states of f-block elements, and also gain practical experience through laboratory work and experiments. They learn about the preparation and properties of f-block compounds, including their reactions with different ligands and their use in catalysis, among other applications.
The course is also interdisciplinary in nature, drawing on concepts from physics, materials science, and engineering. Students learn about the unique physical and chemical properties of f-block elements, which make them useful in a variety of applications such as nuclear reactors, lighting, and medicine.
In addition to theoretical and practical knowledge, the course also emphasizes critical thinking and problem-solving skills. Students are required to analyze and interpret experimental data, apply theoretical concepts to practical problems, and work collaboratively in groups.
Overall, the JEE (Main+Advance) Intermediate Course for f-Block Elements is a demanding and challenging course that requires a strong foundation in chemistry and mathematics. However, it provides students with a deep understanding of the properties and applications of f-block elements, and prepares them for further study and research in this field.
Importance of JEE (Main+Advance) Intermediate Course f-Block Elements
The JEE (Main+Advance) Intermediate Course for f-Block Elements is an important course for students studying chemistry, materials science, and engineering. There are several reasons why this course is important:
- Understanding of the properties and behavior of f-block elements: f-block elements are unique and exhibit a wide range of physical and chemical properties that differ from those of other elements. The course helps students understand the electronic structure, coordination chemistry, magnetic properties, and other properties of these elements.
- Applications in various fields: f-block elements have many important applications in various fields, such as nuclear energy, lighting, catalysis, and medicine. The course provides students with an understanding of the practical applications of these elements and how they can be used in various industries.
- Preparation for higher education: students who complete the JEE (Main+Advance) Intermediate Course for f-Block Elements are well-prepared for higher education in chemistry and materials science. The course provides a strong foundation in the properties and applications of f-block elements, which can be built upon in further study and research.
- Research opportunities: the study of f-block elements is an active area of research, and students who complete the course are well-prepared to pursue research opportunities in this field. They have the knowledge and skills necessary to work with f-block elements and investigate their properties and applications.
Overall, the JEE (Main+Advance) Intermediate Course for f-Block Elements is an important course for students interested in chemistry, materials science, and engineering. It provides a deep understanding of the properties and applications of f-block elements and prepares students for further study and research in this field.
Benefits of JEE (Main+Advance) Intermediate Course f-Block Elements
The JEE (Main+Advance) Intermediate Course for f-Block Elements offers several benefits to students who complete the course:
- Strong foundation in chemistry: the course provides students with a strong foundation in chemistry, which is essential for pursuing higher education in this field. They learn about the electronic structure, coordination chemistry, and other properties of f-block elements, which are important for understanding the behavior of other elements as well.
- Preparation for competitive exams: students who complete the course are well-prepared for competitive exams such as the JEE (Main+Advance), which require a strong understanding of chemistry. The course covers many topics that are part of the JEE syllabus, including chemical bonding, coordination compounds, and redox reactions.
- Career opportunities: the study of f-block elements opens up many career opportunities in various industries, including nuclear energy, lighting, and catalysis. Students who complete the course have the knowledge and skills necessary to pursue careers in these fields.
- Research opportunities: the study of f-block elements is an active area of research, and students who complete the course are well-prepared to pursue research opportunities in this field. They have the knowledge and skills necessary to work with f-block elements and investigate their properties and applications.
- Improved problem-solving skills: the course emphasizes critical thinking and problem-solving skills, which are essential for success in any field. Students learn how to analyze and interpret experimental data, apply theoretical concepts to practical problems, and work collaboratively in groups.
Overall, the JEE (Main+Advance) Intermediate Course for f-Block Elements offers many benefits to students who complete the course. It provides a strong foundation in chemistry, prepares students for competitive exams and career opportunities, and improves their problem-solving skills.
Overview of JEE (Main+Advance) Intermediate Course f-Block Elements
The JEE (Main+Advance) Intermediate Course on f-Block Elements is a course designed for students preparing for the Joint Entrance Examination (JEE), which is the primary entrance examination for admission to engineering colleges in India. The f-Block Elements are a group of elements in the periodic table that have partially filled 4f and 5f orbitals.
The course covers the following topics related to f-Block Elements:
- Introduction to f-Block Elements: This section provides an overview of f-Block Elements and their characteristics. Students will learn about the electronic configuration of f-Block Elements, their oxidation states, and the physical and chemical properties of these elements.
- Lanthanides: This section covers the lanthanides, a group of 14 elements that have partially filled 4f orbitals. Students will learn about the electronic configuration, properties, and uses of these elements.
- Actinides: This section covers the actinides, a group of 14 elements that have partially filled 5f orbitals. Students will learn about the electronic configuration, properties, and uses of these elements.
- Complex Formation: This section covers the formation of complexes between f-Block Elements and ligands. Students will learn about the different types of ligands, the structures of complexes, and the factors that affect complex stability.
- Coordination Chemistry: This section covers coordination chemistry, which is the study of the interaction between metal ions and ligands. Students will learn about the different types of coordination compounds, the nomenclature of coordination compounds, and the isomerism in coordination compounds.
- Extraction of f-Block Elements: This section covers the extraction of f-Block Elements from their ores. Students will learn about the different methods used for extraction, including pyrometallurgical and hydrometallurgical methods.
Overall, the JEE (Main+Advance) Intermediate Course on f-Block Elements is designed to provide students with a comprehensive understanding of the properties and uses of f-Block Elements, as well as their relevance to chemistry and engineering.
Types of JEE (Main+Advance) Intermediate Course f-Block Elements
There are various types of JEE (Main+Advance) Intermediate Courses available for f-Block Elements. Some of the common types are:
- Online Courses: These courses are delivered entirely online, and students can access course materials and lectures at their convenience. Online courses often include video lectures, interactive quizzes, and assignments.
- Classroom Courses: These courses are conducted in a traditional classroom setting, with a teacher delivering lectures and providing guidance to students. Classroom courses may also include group discussions, problem-solving sessions, and practice tests.
- Hybrid Courses: These courses combine online and classroom instruction. Students attend some classes in-person and complete the rest of the coursework online.
- Crash Courses: These courses are designed for students who want to quickly review the material before the exam. Crash courses are typically shorter in duration and cover only the most essential topics.
- Test Series Courses: These courses focus on providing students with practice tests and mock exams. They help students prepare for the JEE by familiarizing them with the exam format and types of questions asked.
- Self-paced Courses: These courses allow students to learn at their own pace, without the pressure of deadlines or schedules. Self-paced courses may include recorded lectures, interactive exercises, and assessments.
Regardless of the type of course, the focus is on providing students with a comprehensive understanding of f-Block Elements and their properties, as well as preparing them for the JEE (Main+Advance) exam.
Structures of JEE (Main+Advance) Intermediate Course f-Block Elements
The structure of JEE (Main+Advance) Intermediate Course for f-Block Elements typically consists of the following components:
- Introduction: This section provides an overview of the course and its objectives. It may also include information on the instructor and the course syllabus.
- Basic Concepts: This section covers the fundamental concepts related to f-Block Elements, including electronic configuration, oxidation states, and physical and chemical properties.
- Lanthanides and Actinides: These sections cover the properties, uses, and applications of lanthanides and actinides.
- Complex Formation: This section covers the formation of complexes between f-Block Elements and ligands, including the types of ligands, the structures of complexes, and the factors that affect complex stability.
- Coordination Chemistry: This section covers coordination chemistry, which is the study of the interaction between metal ions and ligands. It covers the different types of coordination compounds, the nomenclature of coordination compounds, and isomerism in coordination compounds.
- Extraction of f-Block Elements: This section covers the methods used for the extraction of f-Block Elements from their ores.
- Practice Questions and Assignments: Throughout the course, students will have the opportunity to complete practice questions and assignments to reinforce their understanding of the material.
- Mock Tests and Assessments: The course typically concludes with mock tests and assessments to evaluate the students’ knowledge and preparedness for the JEE (Main+Advance) exam.
The course structure may vary depending on the type of course and the institution offering it, but the above components are common to most JEE (Main+Advance) Intermediate Courses for f-Block Elements.
Application of JEE (Main+Advance) Intermediate Course f-Block Elements
The JEE (Main+Advance) Intermediate Course for f-Block Elements has several practical applications, including:
- Engineering: Engineers use f-Block Elements in various applications, such as building materials, electronics, and energy production. The course provides a foundation for understanding the properties of these elements and their applications in engineering.
- Chemical Research: The study of f-Block Elements has applications in chemical research, such as developing new catalysts and understanding the behavior of metal ions in biological systems.
- Pharmaceuticals: Many pharmaceuticals contain f-Block Elements, and the course provides an understanding of the properties of these elements and their interactions with ligands, which is critical for drug development.
- Environmental Science: The study of f-Block Elements is relevant to environmental science, as many of these elements are present in soil, water, and air. Understanding their properties and behavior in the environment is critical for developing strategies to mitigate their impact on human health and the environment.
- Materials Science: F-Block Elements have unique electronic and magnetic properties that make them attractive for use in materials science, such as magnetic data storage and superconductors.
In summary, the JEE (Main+Advance) Intermediate Course for f-Block Elements has practical applications in various fields, including engineering, chemical research, pharmaceuticals, environmental science, and materials science. The course provides students with a foundation for understanding the properties of these elements and their applications in these fields.
Nomenclature of JEE (Main+Advance) Intermediate Course f-Block Elements
The nomenclature of JEE (Main+Advance) Intermediate Course f-Block Elements follows a set of rules established by the International Union of Pure and Applied Chemistry (IUPAC).
- Electron Configuration: The electron configuration of the element is written in the form of noble gas configuration followed by the outermost electrons. For example, the electron configuration of cerium is [Xe]4f1 5d1 6s2.
- Oxidation State: The oxidation state of the element is indicated by a Roman numeral in parentheses following the element name. For example, the common oxidation states of cerium are +3 and +4, so the element is named cerium(III) or cerium(IV).
- Ligands: In complex compounds, the ligands are named first and then followed by the name of the metal, which is indicated by a Roman numeral in parentheses. For example, [Fe(H2O)6]2+ is named hexaaquairon(II) ion, where the ligand is water and the metal is iron in the +2 oxidation state.
- Coordination Number: The coordination number is the number of ligands attached to the central metal ion. The coordination number is indicated by a prefix before the ligand name. For example, [Co(NH3)6]3+ is named hexaamminecobalt(III) ion, where the prefix hexa- indicates the coordination number of 6.
- Isomers: When there are different spatial arrangements of the same atoms, they are called isomers. The prefix cis- or trans- is used to indicate the relative positions of the ligands. For example, cisplatin and transplatin are two different isomers of the same compound, [PtCl2(NH3)2].
In summary, the nomenclature of JEE (Main+Advance) Intermediate Course f-Block Elements follows the IUPAC rules, which include indicating the electron configuration, oxidation state, ligands, coordination number, and isomers. These rules help to provide a systematic and unambiguous naming system for f-Block Elements and their compounds.
Career Opportunities of JEE (Main+Advance) Intermediate Course f-Block Elements
The JEE (Main+Advance) Intermediate Course for f-Block Elements provides students with a strong foundation in the properties and applications of these elements, which can lead to various career opportunities in different fields. Here are some examples:
- Chemical Engineering: Graduates with a background in f-Block Elements can pursue careers in chemical engineering, where they can work on the development of materials, chemical processes, and products that use f-Block Elements.
- Materials Science: The study of f-Block Elements is critical to materials science, which involves the development of new materials with unique properties. Graduates with a background in f-Block Elements can work in materials science research and development, designing new materials for use in various industries.
- Pharmaceuticals: Many pharmaceuticals contain f-Block Elements, and graduates with a background in f-Block Elements can work in pharmaceutical research and development, designing new drugs or improving existing ones.
- Environmental Science: The study of f-Block Elements is relevant to environmental science, and graduates with a background in f-Block Elements can work on developing strategies to mitigate the impact of these elements on human health and the environment.
- Academia: Graduates with a background in f-Block Elements can pursue academic careers in universities and research institutions, conducting research and teaching courses related to f-Block Elements and their applications.
- Government: Graduates with a background in f-Block Elements can work for government agencies, such as the Environmental Protection Agency or the Department of Energy, to develop policies related to the use and disposal of f-Block Elements.
In summary, the JEE (Main+Advance) Intermediate Course for f-Block Elements provides students with a foundation for various career opportunities in chemical engineering, materials science, pharmaceuticals, environmental science, academia, and government. The practical applications of f-Block Elements in these fields offer diverse and exciting opportunities for graduates with this background.