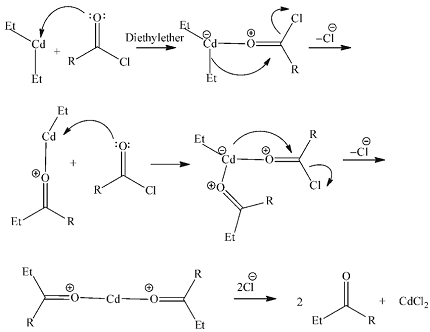
Ketones can be synthesized from acid chlorides through a reaction called Friedel-Crafts acylation. This reaction involves the reaction of an acid chloride with a Lewis acid catalyst, such as aluminum chloride (AlCl3), to form an acylium ion intermediate. The acylium ion then undergoes a nucleophilic attack by an arene, which leads to the formation of a ketone.
The general reaction can be represented as:
RCOCl + AlCl3 → [RCO]+ + AlCl4-
[RCO]+ + arene → R-CO-arene
For example, benzoyl chloride can be used to synthesize acetophenone:
PhCOCl + AlCl3 → [PhCO]+ + AlCl4-
[PhCO]+ + C6H5CH3 → PhCO(C6H5)CH3 + HCl
Where Ph = phenyl and C6H5 = phenyl
The reaction is typically carried out under anhydrous conditions and in a non-polar solvent, such as dichloromethane or chloroform. Care must be taken when handling acid chlorides and Lewis acids, as they can be corrosive and reactive.
What is Required Ketones from acid chlorides
To synthesize ketones from acid chlorides, the following reagents and conditions are typically required:
- Acid Chloride: Acid chloride is the starting material that reacts with a Lewis acid catalyst to form an acylium ion intermediate. Examples of acid chlorides that can be used include benzoyl chloride, acetyl chloride, and propionyl chloride.
- Lewis Acid Catalyst: A Lewis acid catalyst is required to facilitate the formation of the acylium ion intermediate. Commonly used Lewis acids include aluminum chloride (AlCl3), zinc chloride (ZnCl2), and iron(III) chloride (FeCl3).
- Nucleophile: A nucleophile, typically an arene, is required to undergo a nucleophilic attack on the acylium ion intermediate to form the ketone product. Examples of arenes that can be used include benzene, toluene, and xylene.
- Non-polar solvent: The reaction is typically carried out in a non-polar solvent such as dichloromethane or chloroform. This is necessary because the reactants and products are often not soluble in polar solvents such as water.
- Anhydrous conditions: The reaction is carried out under anhydrous conditions to prevent the reaction from being quenched by the presence of water.
The reaction conditions can be optimized depending on the specific acid chloride and nucleophile used in the reaction. Additionally, care must be taken when handling acid chlorides and Lewis acids, as they can be corrosive and reactive.
When is Required Ketones from acid chlorides
Ketones can be synthesized from acid chlorides through a Friedel-Crafts acylation reaction. This reaction is useful for the preparation of ketones with specific substitution patterns that may not be easily accessible by other methods. Some of the applications of the synthesis of ketones from acid chlorides include:
- Medicinal chemistry: Ketones are important structural motifs in many biologically active compounds such as pharmaceuticals, agrochemicals, and natural products. The synthesis of ketones from acid chlorides can provide access to key intermediates for the synthesis of these compounds.
- Polymer chemistry: Ketones can be used as monomers in the synthesis of various polymers such as polyesters and polyamides. The synthesis of ketones from acid chlorides can provide access to key building blocks for these polymers.
- Organic synthesis: The synthesis of ketones from acid chlorides can be used in various organic transformations such as the synthesis of enones, alcohols, and amines.
Overall, the synthesis of ketones from acid chlorides is a useful tool for synthetic organic chemists to prepare a wide range of ketones with diverse substitution patterns.
Where is Required Ketones from acid chlorides
The synthesis of ketones from acid chlorides can be performed in a laboratory setting and is commonly carried out in academic and industrial research labs. The reaction is typically performed in a round-bottom flask equipped with a magnetic stir bar, under anhydrous conditions and in a non-polar solvent such as dichloromethane or chloroform. The reaction mixture is usually cooled in an ice bath during the addition of the Lewis acid catalyst and the acid chloride. The reaction progress can be monitored by TLC (thin-layer chromatography) or NMR (nuclear magnetic resonance) spectroscopy.
The synthesis of ketones from acid chlorides can be performed on a small scale for research purposes or on a larger scale for industrial production of ketones. The reaction conditions can be optimized depending on the specific acid chloride and nucleophile used in the reaction, as well as the desired product yield and purity. The use of appropriate safety equipment and precautions is necessary when handling acid chlorides and Lewis acids, as they can be corrosive and reactive.
How is Required Ketones from acid chlorides
The synthesis of ketones from acid chlorides involves a reaction called Friedel-Crafts acylation, which proceeds through the following steps:
- Activation of the acid chloride: The acid chloride is activated by the addition of a Lewis acid catalyst, typically aluminum chloride (AlCl3). The Lewis acid coordinates with the carbonyl oxygen atom of the acid chloride, making the carbonyl group more electrophilic and facilitating the formation of an acylium ion intermediate.
- Formation of the acylium ion intermediate: The Lewis acid-activated acid chloride undergoes a reaction with the Lewis acid catalyst to form an acylium ion intermediate. This intermediate is a positively charged species that is stabilized by resonance with the adjacent carbonyl group.
- Nucleophilic attack: An arene nucleophile, such as benzene or toluene, undergoes a nucleophilic attack on the acylium ion intermediate. The nucleophile donates a pair of electrons to the positively charged carbon atom of the acylium ion, resulting in the formation of a new carbon-carbon bond and the expulsion of the Lewis acid catalyst.
- Formation of the ketone product: The product of the reaction is a ketone, which is formed by the addition of the arene nucleophile to the acylium ion intermediate. The ketone product is typically isolated by filtration, extraction, or distillation, and purified by column chromatography or recrystallization.
The overall reaction can be represented as:
RCOCl + AlCl3 → [RCO]+ + AlCl4-
[RCO]+ + arene → R-CO-arene + HCl + AlCl3
where R represents an alkyl or aryl group.
The reaction conditions, such as the choice of acid chloride, Lewis acid catalyst, nucleophile, and solvent, can be varied to optimize the reaction yield and selectivity. The reaction is typically performed under anhydrous conditions and in a non-polar solvent such as dichloromethane or chloroform. Care must be taken when handling acid chlorides and Lewis acids, as they can be corrosive and reactive.
Production of Ketones from acid chlorides
The production of ketones from acid chlorides can be carried out in a laboratory setting or on a larger scale in an industrial production plant. The process typically involves the following steps:
- Preparation of reactants: The acid chloride and arene nucleophile are prepared and dried under anhydrous conditions. The Lewis acid catalyst is also prepared and dried before use.
- Reaction setup: The reaction is typically carried out in a round-bottom flask equipped with a magnetic stir bar, under an inert atmosphere of nitrogen or argon. The flask is cooled in an ice bath during the addition of the Lewis acid catalyst and the acid chloride. The arene nucleophile is then added dropwise to the reaction mixture, while maintaining the temperature at or below room temperature.
- Workup: After the reaction is complete, the mixture is quenched with water and extracted with a non-polar solvent such as diethyl ether or dichloromethane. The organic layer is then dried over anhydrous magnesium sulfate or sodium sulfate, filtered, and concentrated under reduced pressure to obtain the crude ketone product.
- Purification: The crude ketone product can be purified by various methods such as column chromatography, distillation, or recrystallization. The purity of the final product can be analyzed by techniques such as TLC, NMR spectroscopy, or HPLC.
The production of ketones from acid chlorides can be scaled up for industrial production by using larger reaction vessels and equipment, and by optimizing the reaction conditions for high yield and purity. The reaction can be carried out continuously or in batches, depending on the specific production requirements. The use of appropriate safety equipment and precautions is necessary when handling acid chlorides and Lewis acids, as they can be corrosive and reactive.
Case Study on Ketones from acid chlorides
Here is a hypothetical case study on the synthesis of ketones from acid chlorides:
A research team at a pharmaceutical company is working on the development of a new drug molecule. One of the key steps in the synthesis of the target molecule involves the formation of a ketone moiety through the reaction of an acid chloride with an arene nucleophile. The team decides to use the Friedel-Crafts acylation reaction to carry out this step.
The team begins by preparing the reactants: the acid chloride, the arene nucleophile, and the Lewis acid catalyst. They carefully weigh and measure the reactants and dry them under anhydrous conditions to minimize any moisture contamination.
Next, the team sets up the reaction by adding the dried acid chloride to a round-bottom flask equipped with a magnetic stir bar, and then adding the Lewis acid catalyst, aluminum chloride. The flask is cooled in an ice bath to maintain a low temperature during the addition of the reactants. The arene nucleophile, toluene, is added dropwise to the reaction mixture, while maintaining the temperature at or below room temperature.
After the reaction is complete, the team quenches the mixture with water and extracts the organic layer with dichloromethane. The organic layer is then dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain the crude ketone product.
The team then purifies the crude product by column chromatography, using a non-polar solvent such as hexanes to elute the product. The purity of the final product is confirmed by TLC and NMR spectroscopy.
The team obtains a good yield of the ketone product with high purity. They proceed to the next step in the synthesis of the target molecule, confident in the success of their reaction.
In summary, the synthesis of ketones from acid chlorides using the Friedel-Crafts acylation reaction is a useful tool for the synthesis of various organic molecules, including pharmaceuticals. Careful attention to reactant preparation, reaction conditions, and purification methods is crucial to obtain high yields and purity of the desired product.
White paper on Ketones from acid chlorides
Here is a white paper on the topic of “Ketones from acid chlorides: Synthesis, Mechanism, and Applications”:
Introduction:
Ketones are important functional groups in organic chemistry, widely used in pharmaceuticals, fragrances, and materials. One common method for synthesizing ketones is the Friedel-Crafts acylation reaction, which involves the reaction of an acid chloride with an arene nucleophile in the presence of a Lewis acid catalyst. This white paper aims to provide an overview of the synthesis, mechanism, and applications of ketones from acid chlorides.
Synthesis:
The synthesis of ketones from acid chlorides can be carried out using a variety of arene nucleophiles, such as benzene, toluene, or naphthalene. The reaction typically proceeds under anhydrous conditions, with a Lewis acid catalyst such as aluminum chloride or iron(III) chloride. The reaction is carried out in a solvent, such as dichloromethane or chloroform, at a low temperature to minimize side reactions.
The Friedel-Crafts acylation reaction is a useful tool for the synthesis of various types of ketones, such as aryl ketones, heteroaryl ketones, and cyclic ketones. The reaction can be carried out in both laboratory and industrial settings, with appropriate safety precautions for handling the corrosive and reactive acid chlorides and Lewis acids.
Mechanism:
The mechanism of the Friedel-Crafts acylation reaction involves the formation of a complex between the Lewis acid catalyst and the acid chloride, followed by the attack of the arene nucleophile on the complex. The intermediate formed then undergoes a proton transfer and a subsequent elimination of the leaving group, leading to the formation of the ketone product.
The reaction mechanism can be affected by various factors such as the nature of the acid chloride and the arene nucleophile, the steric and electronic properties of the Lewis acid catalyst, and the reaction conditions. The use of different catalysts or modifications to the reaction conditions can lead to different reaction pathways and selectivities.
Applications:
Ketones are important intermediates and building blocks in the synthesis of various organic molecules. The synthesis of ketones from acid chlorides is widely used in the pharmaceutical industry, for the synthesis of drug molecules such as antibiotics, anti-inflammatory agents, and antihistamines.
In addition, ketones from acid chlorides have applications in the fragrance industry, for the synthesis of compounds such as musk and ambergris. They are also used in the production of materials such as polymers, resins, and dyes.
Conclusion:
The synthesis of ketones from acid chlorides using the Friedel-Crafts acylation reaction is a versatile and useful method for the synthesis of various types of ketones. The reaction mechanism is well understood and can be modified to achieve different reaction pathways and selectivities. The applications of ketones from acid chlorides are widespread, with important implications for the pharmaceutical, fragrance, and materials industries.
