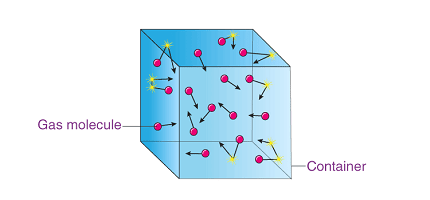
The kinetic theory of gases is a model that explains the behavior of gases in terms of the motion of their individual particles. The theory is based on the following assumptions:
- Gas particles are in constant, random motion.
- Gas particles are infinitely small and have negligible volume.
- Gas particles are elastic and do not lose energy during collisions with other particles or with the walls of the container.
- The average kinetic energy of gas particles is proportional to the temperature of the gas.
From these assumptions, the kinetic theory of gases explains several properties of gases, such as:
- Pressure: The pressure exerted by a gas on the walls of its container is due to the collisions of gas particles with the walls. The more collisions there are, the greater the pressure.
- Volume: Gas particles have negligible volume, so they can be compressed into smaller volumes. The volume of a gas is therefore determined by the size of its container.
- Temperature: The kinetic energy of gas particles is directly proportional to the temperature of the gas. As the temperature increases, the particles move faster and collide more frequently, resulting in an increase in pressure.
- Diffusion and effusion: Gas particles diffuse from high concentration to low concentration areas and effuse through small openings at a rate determined by their mass and velocity.
Overall, the kinetic theory of gases provides a useful framework for understanding the behavior of gases and can be applied in many fields, including chemistry, physics, and engineering.
What is Required Kinetic theory of gases
The kinetic theory of gases requires four main assumptions to explain the behavior of gases:
- Gas particles are in constant, random motion: The first assumption is that the particles in a gas are in constant motion, moving in random directions and colliding with each other and the walls of the container. This motion is due to the kinetic energy of the particles.
- Gas particles are small and have negligible volume: The second assumption is that gas particles are so small that they have no significant volume compared to the container they are in. This means that the particles can move freely within the container and that the overall volume of the gas is determined by the volume of the container.
- Gas particles are elastic and do not lose energy during collisions: The third assumption is that gas particles are perfectly elastic and do not lose energy when they collide with each other or with the walls of the container. This means that the total energy of the gas particles is conserved, and any energy lost during a collision is immediately transferred to another particle.
- The average kinetic energy of gas particles is proportional to the temperature: The fourth assumption is that the average kinetic energy of gas particles is directly proportional to the temperature of the gas. This means that at higher temperatures, the gas particles move faster and collide more frequently, resulting in an increase in pressure.
Overall, these assumptions form the basis of the kinetic theory of gases and allow us to explain the behavior of gases in terms of the motion of their individual particles.
Who is Required Kinetic theory of gases
The kinetic theory of gases was developed by a number of scientists over the course of the 19th century, including James Clerk Maxwell, Rudolf Clausius, and Ludwig Boltzmann. These scientists used mathematical models to describe the behavior of gases based on the motion of individual particles. In particular, Maxwell developed a distribution of velocities for gas particles, known as the Maxwell-Boltzmann distribution, which is still widely used today to describe the behavior of gases.
The kinetic theory of gases has been applied in many fields, including chemistry, physics, and engineering. It is used to explain a wide range of phenomena, such as the diffusion of gases, the behavior of gases under different temperatures and pressures, and the properties of gas mixtures. The theory is also used to design and optimize gas-powered technologies, such as internal combustion engines and gas turbines.
Overall, the kinetic theory of gases is an important scientific theory that has had a significant impact on our understanding of the behavior of gases and its practical applications.
When is Required Kinetic theory of gases
The kinetic theory of gases is required whenever we want to understand the behavior of gases, particularly at the molecular level. It is used to explain a wide range of phenomena, such as the pressure, temperature, volume, and diffusion of gases.
For example, the kinetic theory of gases can help us understand why the pressure of a gas increases as the temperature increases. As the temperature increases, the average kinetic energy of the gas particles also increases, causing the particles to move faster and collide with the walls of the container more frequently, resulting in an increase in pressure.
The kinetic theory of gases is also used in engineering applications, such as designing combustion engines or understanding the behavior of gas mixtures. In these applications, the theory can be used to optimize the performance of gas-powered technologies and to ensure safe and efficient operation.
Overall, the kinetic theory of gases is an important tool for understanding the behavior of gases in a variety of contexts and is required whenever we want to understand the behavior of gases at a molecular level.
Where is Required Kinetic theory of gases
The kinetic theory of gases is required in many fields of science and engineering where the behavior of gases needs to be understood. Some common areas where the kinetic theory of gases is applied include:
- Chemistry: In chemistry, the kinetic theory of gases is used to explain the behavior of gases in chemical reactions, to understand the properties of gases in different phases, and to design and optimize gas-based processes.
- Physics: In physics, the kinetic theory of gases is used to explain the behavior of gases in various physical systems, such as thermodynamics, fluid dynamics, and plasma physics.
- Engineering: In engineering, the kinetic theory of gases is used to design and optimize gas-based technologies, such as combustion engines, gas turbines, and fuel cells.
- Atmospheric science: In atmospheric science, the kinetic theory of gases is used to understand the behavior of gases in the Earth’s atmosphere, including the behavior of air pollution, greenhouse gases, and the ozone layer.
Overall, the kinetic theory of gases is required wherever gases are involved, and it plays a crucial role in understanding and optimizing a wide range of scientific and engineering applications.
How is Required Kinetic theory of gases
The kinetic theory of gases is based on the fundamental idea that gases consist of a large number of small particles, such as atoms or molecules, that are in constant motion. This motion is driven by the kinetic energy of the particles and is affected by a number of factors, including temperature, pressure, and volume.
The kinetic theory of gases makes several key assumptions about the behavior of gas particles:
- Gas particles are in constant, random motion.
- Gas particles are small and have negligible volume.
- Gas particles are elastic and do not lose energy during collisions.
- The average kinetic energy of gas particles is proportional to the temperature.
Using these assumptions, the kinetic theory of gases can be used to derive a number of important relationships between the properties of gases, such as the ideal gas law, which relates pressure, volume, temperature, and the number of gas particles.
In addition, the kinetic theory of gases can be used to explain a wide range of phenomena, such as the diffusion of gases, the behavior of gases under different temperatures and pressures, and the properties of gas mixtures.
Overall, the kinetic theory of gases is a powerful tool for understanding the behavior of gases at a molecular level, and it has applications in many fields of science and engineering.
Case Study on Kinetic theory of gases
One example of the application of the kinetic theory of gases can be seen in the design of combustion engines, such as those used in cars and trucks. Combustion engines rely on the controlled explosion of a fuel-air mixture to generate mechanical energy, which is used to power the vehicle. The kinetic theory of gases plays an important role in understanding the behavior of gases within the engine and optimizing its performance.
One important factor in the design of combustion engines is the air-fuel ratio, which determines the amount of air and fuel that is mixed together before being ignited. The ideal air-fuel ratio depends on the type of fuel being used and the design of the engine. If the air-fuel ratio is too low, the engine will run inefficiently and produce excess pollutants. If the air-fuel ratio is too high, the engine may not run at all.
The kinetic theory of gases can be used to predict the behavior of the air-fuel mixture within the engine, taking into account factors such as temperature, pressure, and the size and velocity of individual gas particles. This information can be used to optimize the design of the combustion chamber, fuel injection system, and other components of the engine to ensure efficient and reliable operation.
For example, the kinetic theory of gases can be used to understand the process of fuel injection, where fuel is sprayed into the combustion chamber in small droplets. The droplets are quickly vaporized by the high temperature within the chamber, and the resulting gas mixture is ignited by a spark plug. By modeling the behavior of the individual gas particles within the chamber, engineers can optimize the size and timing of the fuel injection to maximize efficiency and minimize pollutant emissions.
Overall, the kinetic theory of gases plays an important role in the design and optimization of combustion engines, which are critical to many aspects of modern society. By understanding the behavior of gases at a molecular level, engineers can create more efficient and environmentally-friendly engines that help to meet the world’s energy needs.
White paper on Kinetic theory of gases
Introduction:
The kinetic theory of gases is a fundamental concept in the field of thermodynamics and is based on the idea that gases are made up of a large number of small particles that are in constant, random motion. This theory provides a molecular-level understanding of the behavior of gases and is used in a wide range of scientific and engineering applications. In this white paper, we will explore the principles of the kinetic theory of gases, its applications, and its implications for our understanding of the physical world.
The Basics of the Kinetic Theory of Gases:
The kinetic theory of gases is based on several key assumptions about the behavior of gas particles. First, it assumes that gas particles are in constant, random motion, colliding with each other and with the walls of their container. Second, it assumes that gas particles are small and have negligible volume, so they can be treated as point masses. Third, it assumes that gas particles are elastic and do not lose energy during collisions, so the total kinetic energy of the gas is conserved. Finally, it assumes that the average kinetic energy of gas particles is proportional to the temperature of the gas.
From these assumptions, the kinetic theory of gases can be used to derive a number of important relationships between the properties of gases, such as the ideal gas law, which relates pressure, volume, temperature, and the number of gas particles. The ideal gas law is an important tool in engineering and science and is used to describe the behavior of gases under a wide range of conditions.
Applications of the Kinetic Theory of Gases:
The kinetic theory of gases has a wide range of applications in science and engineering. One important application is in the design of combustion engines, where the controlled explosion of a fuel-air mixture generates mechanical energy that is used to power a vehicle. The kinetic theory of gases is used to understand the behavior of the gas mixture within the engine and optimize its performance.
Another important application of the kinetic theory of gases is in the field of atmospheric science, where it is used to understand the behavior of gases in the Earth’s atmosphere. This includes the behavior of air pollution, greenhouse gases, and the ozone layer, which are critical factors in climate change and environmental policy.
The kinetic theory of gases is also used in chemistry and physics to understand the behavior of gases in chemical reactions, to explain the properties of gases in different phases, and to design and optimize gas-based processes.
Implications for Our Understanding of the Physical World:
The kinetic theory of gases has important implications for our understanding of the physical world. It provides a molecular-level understanding of the behavior of gases and helps us to understand the macroscopic properties of gases, such as their pressure, volume, and temperature. It also helps us to understand the behavior of gases in different phases, such as liquids and solids, and to explain a wide range of physical phenomena, such as diffusion, thermal conductivity, and viscosity.
Conclusion:
In conclusion, the kinetic theory of gases is a fundamental concept in the field of thermodynamics and provides a molecular-level understanding of the behavior of gases. It has a wide range of applications in science and engineering and has important implications for our understanding of the physical world. As we continue to develop new technologies and address the challenges of the 21st century, the kinetic theory of gases will remain an essential tool for scientists and engineers.
