The Kolbe reaction, also known as the Kolbe electrolysis or Kolbe-Schmitt reaction, is a chemical reaction in which a carboxylic acid is produced by the electrolysis of a solution of its salts, primarily the sodium or potassium salts. The reaction was discovered by Hermann Kolbe in 1845.
In the Kolbe reaction, an alkali metal salt of a carboxylic acid is electrolyzed in an aqueous or alcoholic solution. At the anode, the salt is oxidized to form a carboxylate radical, which then reacts with another carboxylate radical to form a dimer. The dimer undergoes decarboxylation to give the corresponding alkane and carbon dioxide. The overall reaction can be represented as follows:
2 RCOO- → R-R + 2 CO2 + 2 e-
where R represents an alkyl or aryl group.
The Kolbe reaction is an important method for the preparation of carboxylic acids, especially those with odd numbers of carbon atoms. It has also been used for the synthesis of other organic compounds, such as phenols, benzene derivatives, and ketones.
What is Required Kolbe reaction
The Required Kolbe Reaction is a modification of the Kolbe reaction, in which a specific organic compound is produced by the reaction. In this case, the reactants used in the reaction are chosen based on the desired organic product.
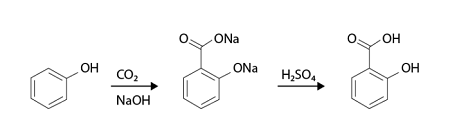
For example, in the synthesis of salicylic acid, the Required Kolbe Reaction can be used. Salicylic acid is synthesized by the Kolbe reaction of sodium phenoxide with carbon dioxide, followed by acidification of the resulting sodium salicylate. The overall reaction can be represented as follows:
C6H5ONa + CO2 → C6H4(CO2Na)OH
C6H4(CO2Na)OH + HCl → C6H4(CO2H)OH + NaCl
In this reaction, sodium phenoxide is used as a reactant, which reacts with carbon dioxide to form the intermediate sodium salicylate. Upon acidification, sodium salicylate is converted to salicylic acid.
The Required Kolbe Reaction is a versatile reaction that can be used for the synthesis of a variety of organic compounds. It is a useful tool for organic chemists to design and synthesize specific organic molecules.
When is Required Kolbe reaction
The Required Kolbe Reaction is used when a specific organic compound is required to be synthesized. The reactants and conditions for the reaction are chosen based on the desired organic product. This reaction is commonly used in organic synthesis to prepare a wide range of organic compounds, including carboxylic acids, phenols, and other organic derivatives.
The Required Kolbe Reaction is often used when traditional synthetic methods are not practical or efficient. For example, the synthesis of some complex organic molecules requires multiple reaction steps, which can be time-consuming and challenging. The Required Kolbe Reaction can be used to simplify the synthesis process by directly converting simpler reactants into the desired organic product in a single step.
Overall, the Required Kolbe Reaction is a useful tool in organic chemistry for designing and synthesizing specific organic compounds in a straightforward and efficient manner.
Where is Required Kolbe reaction
The Required Kolbe Reaction is a versatile organic reaction that can be performed in a variety of laboratory settings. It can be carried out in both aqueous and non-aqueous solvents, depending on the choice of reactants and the desired organic product.
The reaction is typically carried out using an electrolytic cell, which consists of two electrodes immersed in the reactant solution. The anode is typically made of platinum or graphite, and the cathode can be made of a variety of materials, such as stainless steel or mercury.
The reaction conditions for the Required Kolbe Reaction can vary depending on the choice of reactants and the desired organic product. For example, the reaction may require heating or cooling to achieve the desired reaction rate or product yield. In addition, the reaction may require the use of a specific catalyst or electrolyte to facilitate the reaction.
Overall, the Required Kolbe Reaction can be performed in a variety of laboratory settings, and the specific conditions can be tailored to suit the needs of the experiment.
How is Required Kolbe reaction
The Required Kolbe Reaction is a type of organic reaction that involves the electrolysis of an electrolyte solution containing a specific organic compound to produce a desired organic product. The reaction involves the use of an electrolytic cell that consists of two electrodes (an anode and a cathode) immersed in the electrolyte solution. The anode is typically made of platinum or graphite, while the cathode can be made of a variety of materials, such as stainless steel or mercury.
The reaction proceeds in several steps. First, the electrolyte is dissociated into its constituent ions in the presence of an electric current. Next, the anode reacts with the negatively charged ions, which results in the formation of free radicals. These radicals then react with other radicals or ions to form intermediate products. Finally, the desired organic product is obtained by isolating and purifying the intermediate product.
The specific conditions of the reaction can vary depending on the choice of reactants and the desired organic product. For example, the reaction may require a specific temperature or pH, or the use of a catalyst or electrolyte to facilitate the reaction. The overall efficiency of the reaction can be optimized by adjusting the reaction conditions and controlling the reaction parameters.
Overall, the Required Kolbe Reaction is a versatile organic reaction that can be used to synthesize a wide range of organic compounds. It is an important tool for organic chemists to design and synthesize specific organic molecules.
Production of Kolbe reaction
The Kolbe reaction is a chemical reaction that involves the electrolysis of a salt of a carboxylic acid to produce the corresponding carboxylic acid. The production of Kolbe reaction involves several steps, which are described below:
- Preparation of the electrolytic cell: The electrolytic cell consists of two electrodes, an anode and a cathode, which are connected to a power source. The anode is typically made of platinum or graphite, while the cathode can be made of a variety of materials, such as stainless steel or mercury. The cell is filled with a solution of the salt of the carboxylic acid to be electrolyzed.
- Electrolysis: When an electric current is passed through the cell, the salt dissociates into its constituent ions. At the anode, the negatively charged ions are oxidized to form carboxylate radicals, which then react with other radicals to form dimeric species. The dimer undergoes decarboxylation to give the corresponding alkane and carbon dioxide, which is evolved as a gas.
2 RCOO- → R-R + 2 CO2 + 2 e-
- Acidification: After the electrolysis, the resulting carboxylate product is typically acidified to give the corresponding carboxylic acid. This is usually done by adding a strong acid, such as hydrochloric acid, to the reaction mixture. The carboxylic acid precipitates out of solution and can be isolated by filtration or other purification techniques.
Overall, the production of Kolbe reaction involves the use of an electrolytic cell to electrolyze a salt of a carboxylic acid to produce the corresponding carboxylic acid. The reaction is an important method for the preparation of carboxylic acids and has been used for the synthesis of other organic compounds as well.
Case Study on Kolbe reaction
One example of the use of Kolbe reaction is the synthesis of salicylic acid, which is a common analgesic and antipyretic medication. Salicylic acid is also used in the manufacture of other pharmaceuticals, dyes, and fragrances.
The synthesis of salicylic acid via the Kolbe reaction involves the electrolysis of sodium salicylate in aqueous solution. The reaction proceeds as follows:
2 NaC7H5O3 (sodium salicylate) → C14H10O4 (salicylic acid) + Na2CO3 (sodium carbonate)
In this reaction, the sodium salicylate is oxidized at the anode to form salicylate radicals, which then react with other radicals to form dimeric species. The dimer undergoes decarboxylation to give the corresponding alkene and carbon dioxide, which is evolved as a gas. The resulting salicylic acid is then obtained by acidification of the reaction mixture using hydrochloric acid, which causes the salicylic acid to precipitate out of solution.
The Kolbe reaction is a useful method for the synthesis of salicylic acid, as it is a simple and efficient way to convert sodium salicylate into salicylic acid in a single step. The reaction can be performed on a large scale and is suitable for industrial production of salicylic acid.
In addition to the synthesis of salicylic acid, the Kolbe reaction has been used in the synthesis of a wide range of other organic compounds, including carboxylic acids, phenols, and other organic derivatives. It is an important tool for organic chemists to design and synthesize specific organic molecules, and its versatility and efficiency make it a valuable technique for industrial chemical production.
White paper on Kolbe reaction
Introduction:
The Kolbe reaction is a well-known organic reaction that involves the electrolysis of a salt of a carboxylic acid to produce the corresponding carboxylic acid. The reaction was first discovered by Hermann Kolbe in 1848 and has since become an important tool for organic chemists in the synthesis of a wide range of organic compounds. In this white paper, we will discuss the Kolbe reaction, its mechanism, applications, and future developments.
Mechanism:
The Kolbe reaction proceeds in several steps. First, the electrolyte is dissociated into its constituent ions in the presence of an electric current. Next, the anode reacts with the negatively charged ions, which results in the formation of free radicals. These radicals then react with other radicals or ions to form intermediate products. Finally, the desired organic product is obtained by isolating and purifying the intermediate product.
Applications:
The Kolbe reaction is a versatile organic reaction that can be used to synthesize a wide range of organic compounds. It is an important tool for organic chemists to design and synthesize specific organic molecules. Some of the notable applications of the Kolbe reaction include:
- Synthesis of salicylic acid: The Kolbe reaction is commonly used in the industrial production of salicylic acid, which is an important pharmaceutical and chemical intermediate.
- Synthesis of other carboxylic acids: The Kolbe reaction has been used to synthesize a wide range of other carboxylic acids, including fatty acids, benzoic acid, and phenylacetic acid.
- Synthesis of phenols: The Kolbe reaction has also been used to synthesize phenols and their derivatives, including resorcinol and hydroquinone.
Future Developments:
The Kolbe reaction has been studied extensively since its discovery, and ongoing research continues to explore its potential for new applications and developments. Some areas of future development in the Kolbe reaction include:
- Development of new electrolytes: The choice of electrolyte can have a significant impact on the efficiency and selectivity of the Kolbe reaction. Research continues to investigate new electrolytes that can improve the reaction yield and reduce waste.
- Development of new catalysts: The use of catalysts can significantly improve the efficiency and selectivity of the Kolbe reaction. Ongoing research is exploring the use of new catalysts, including enzymes and metal complexes, to optimize the reaction conditions.
- Exploration of new reaction conditions: The Kolbe reaction can be performed under a wide range of conditions, including temperature, pH, and pressure. Ongoing research is exploring new reaction conditions that can improve the efficiency and selectivity of the reaction.
Conclusion:
The Kolbe reaction is a versatile and efficient method for the synthesis of a wide range of organic compounds. Its versatility and efficiency make it a valuable tool for both academic and industrial research. Ongoing research continues to explore the potential for new applications and developments in the Kolbe reaction, which will further enhance its utility and importance in organic chemistry.