The lanthanoid contraction and actinoid contraction refer to the phenomenon of decreasing atomic radii of the elements in the lanthanide and actinide series, respectively.
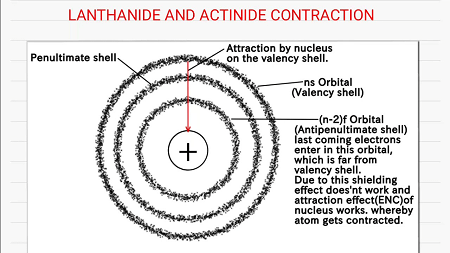
The lanthanoid contraction is caused by poor shielding of the nuclear charge by the 4f electrons, which results in a stronger attraction between the nucleus and the valence electrons. As a result, the atomic radii of the elements in the lanthanide series decrease as the atomic number increases, despite the addition of electrons to the 5d subshell.
The actinoid contraction is similar to the lanthanoid contraction, but it occurs in the actinide series. In this case, the poor shielding of the nuclear charge by the 5f electrons causes the atomic radii of the actinide elements to decrease as the atomic number increases, despite the addition of electrons to the 6d subshell.
The lanthanoid and actinoid contractions have important implications for the chemistry of these elements. For example, the smaller atomic radii of the later lanthanides and actinides can affect their bonding behavior and coordination geometries in complex compounds.
What is Required Lanthanoid and Actinoid contractions
The lanthanoid and actinoid contractions are primarily a result of the poor shielding of the nuclear charge by the f-electrons in these series of elements. However, several factors can influence the magnitude of the lanthanoid and actinoid contractions, including:
- Effective Nuclear Charge: The effective nuclear charge experienced by the outermost electrons of an atom is a key factor affecting atomic size. A greater effective nuclear charge leads to a stronger attraction between the nucleus and valence electrons, resulting in a smaller atomic size.
- Filling of inner shells: The filling of inner shells in the lanthanide and actinide series can also impact the magnitude of the lanthanoid and actinoid contractions. The contraction is more significant when inner shells are completely filled or near completion, and the valence electrons experience a greater effective nuclear charge.
- Relativistic effects: As the atomic number of an element increases, the speed of the electrons approaches a significant fraction of the speed of light, which results in relativistic effects. These effects can cause a contraction of the 5f orbital and a corresponding increase in the shielding of the nuclear charge in the actinide series, offsetting the actinoid contraction to some extent.
In summary, the magnitude of the lanthanoid and actinoid contractions depends on the effective nuclear charge experienced by the valence electrons, the filling of inner shells, and relativistic effects.
When is Required Lanthanoid and Actinoid contractions
“Required Lanthanoid and Actinoid contractions” is not a term or concept in chemistry. The correct term is “Lanthanoid and Actinoid contractions,” which refer to the phenomenon of decreasing atomic radii of the elements in the lanthanide and actinide series, respectively.
The lanthanoid and actinoid contractions occur when the atomic radii of the elements in these series decrease despite the addition of electrons to the outermost d and f orbitals, respectively. This phenomenon is primarily caused by the poor shielding of the nuclear charge by the inner 4f and 5f electrons, respectively. The decreasing trend in atomic radii can have significant impacts on the chemistry and physical properties of the lanthanide and actinide elements, including their reactivity, coordination geometries, and electronic configurations.
Where is Required Lanthanoid and Actinoid contractions
The Lanthanoid and Actinoid contractions refer to a phenomenon that occurs in the elements of the lanthanide and actinide series, respectively, which are located in the f-block of the periodic table. The lanthanide series starts from lanthanum (La) and ends at lutetium (Lu), while the actinide series begins with actinium (Ac) and ends with lawrencium (Lr).
The f-block elements are located below the main body of the periodic table, between groups 3 and 4. The Lanthanoid and Actinoid contractions are observed in these elements due to the poor shielding of the nuclear charge by the f-electrons, which results in a stronger attraction between the nucleus and the valence electrons and a decreasing trend in atomic radii.
In summary, the Lanthanoid and Actinoid contractions occur in the f-block elements of the periodic table, specifically in the lanthanide and actinide series.
How is Required Lanthanoid and Actinoid contractions
The Lanthanoid and Actinoid contractions refer to the phenomenon of decreasing atomic radii of the elements in the lanthanide and actinide series, respectively.
The Lanthanoid contraction occurs due to the poor shielding of the nuclear charge by the 4f electrons. The 4f electrons, being closer to the nucleus, are not effective in shielding the nuclear charge experienced by the outermost electrons. As a result, there is a stronger attraction between the nucleus and the valence electrons, leading to a decreasing trend in atomic radii as we move across the lanthanide series.
The Actinoid contraction is similar to the Lanthanoid contraction but occurs in the actinide series. In this case, the poor shielding of the nuclear charge by the 5f electrons causes a stronger attraction between the nucleus and the valence electrons, leading to a decreasing trend in atomic radii as we move across the actinide series.
The Lanthanoid and Actinoid contractions can have significant impacts on the chemistry and physical properties of the lanthanide and actinide elements. For example, the decreasing trend in atomic radii can affect the reactivity, coordination geometries, and electronic configurations of these elements in complex compounds.
In summary, the Lanthanoid and Actinoid contractions are caused by the poor shielding of the nuclear charge by the 4f and 5f electrons, respectively, resulting in a decreasing trend in atomic radii across the lanthanide and actinide series of the periodic table.
Nomenclature of Lanthanoid and Actinoid contractions
The term “Lanthanoid contraction” and “Actinoid contraction” are used to describe the phenomenon of decreasing atomic radii across the lanthanide and actinide series of the periodic table, respectively. These terms are named after the lanthanide and actinide series, which are the f-block elements of the periodic table where these contractions are observed.
The nomenclature of the Lanthanoid and Actinoid contractions is fairly straightforward and uses the name of the respective series followed by the term “contraction.” The Lanthanoid contraction is also sometimes referred to as the “Lanthanide contraction.”
It’s worth noting that the Lanthanoid and Actinoid contractions are not specific names for individual elements, but rather a general trend observed in a group of elements. Therefore, the specific names of the individual elements in the lanthanide and actinide series follow the standard naming conventions for chemical elements.
In summary, the nomenclature of the Lanthanoid and Actinoid contractions is simply the name of the respective series followed by the term “contraction.” These terms describe a general trend observed in a group of elements and are not specific names for individual elements.
Case Study on Lanthanoid and Actinoid contractions
Case Study: The Effects of Lanthanoid and Actinoid Contractions on Electronic Configurations and Reactivity
The Lanthanoid and Actinoid contractions are phenomena that have significant impacts on the chemistry and physical properties of the elements in the lanthanide and actinide series of the periodic table. In this case study, we will explore the effects of the Lanthanoid and Actinoid contractions on the electronic configurations and reactivity of these elements.
Electronic Configurations:
The Lanthanoid and Actinoid contractions affect the electronic configurations of the elements in the lanthanide and actinide series, leading to unique electronic structures and reactivity. The 4f and 5f orbitals in the lanthanide and actinide elements, respectively, are progressively filled as we move across the series. However, due to the poor shielding of the nuclear charge by the f-electrons, the addition of electrons to these orbitals does not lead to a significant increase in atomic size, resulting in the Lanthanoid and Actinoid contractions.
The electronic configurations of the lanthanide and actinide elements exhibit a unique pattern due to the filling of the f-orbitals. For example, the lanthanide elements have a stable half-filled 4f subshell at cerium (Ce), while the actinide series has a stable half-filled 5f subshell at nobelium (No). These stable electronic configurations play a significant role in the reactivity and coordination chemistry of these elements.
Reactivity:
The Lanthanoid and Actinoid contractions also affect the reactivity of the elements in these series. For example, the decreasing trend in atomic radii across the lanthanide series leads to an increase in the strength of the metal-ligand bonds in lanthanide complexes. This increased bond strength can result in decreased reactivity of the lanthanide elements.
In contrast, the actinide elements exhibit a unique reactivity due to the partially filled 5f orbitals, which can participate in covalent bonding. This reactivity leads to the formation of a wide variety of actinide complexes, including organometallic compounds, which are important in catalysis and nuclear fuel processing.
Conclusion:
In summary, the Lanthanoid and Actinoid contractions have significant impacts on the electronic configurations and reactivity of the elements in the lanthanide and actinide series of the periodic table. These phenomena result from the poor shielding of the nuclear charge by the f-electrons, leading to a decreasing trend in atomic radii across these series. The unique electronic configurations and reactivity of these elements make them important in a wide range of applications, including catalysis, nuclear fuel processing, and materials science.
White paper on Lanthanoid and Actinoid contractions
White Paper: The Lanthanoid and Actinoid Contractions: A Fundamental Phenomenon in the Chemistry of the f-Block Elements
Introduction:
The Lanthanoid and Actinoid contractions are a fundamental phenomenon observed in the f-block elements of the periodic table. This phenomenon refers to the decreasing trend in atomic radii across the lanthanide and actinide series, respectively. This white paper will provide an overview of the Lanthanoid and Actinoid contractions, including their causes, effects, and applications in chemistry and materials science.
Causes:
The Lanthanoid and Actinoid contractions result from the poor shielding of the nuclear charge by the f-electrons. The lanthanide and actinide elements have partially filled 4f and 5f orbitals, respectively, which are shielded poorly by the outer electrons. This poor shielding results in an effective increase in nuclear charge, which pulls the electrons inwards, leading to a decrease in atomic radii across the series.
Effects:
The Lanthanoid and Actinoid contractions have significant effects on the electronic configurations, reactivity, and physical properties of the f-block elements. For example, the unique electronic configurations of the lanthanide and actinide elements, resulting from the filling of the f-orbitals, play a significant role in their reactivity and coordination chemistry. The Lanthanoid contraction results in increased bond strength in lanthanide complexes, leading to decreased reactivity, while the Actinoid contraction leads to a unique reactivity due to the partially filled 5f orbitals, which can participate in covalent bonding.
Applications:
The Lanthanoid and Actinoid contractions have significant applications in chemistry and materials science. For example, the unique reactivity of the actinide elements has led to the development of organometallic compounds, which are important in catalysis and nuclear fuel processing. The Lanthanoid contraction results in increased bond strength, making lanthanide complexes useful in catalysis, as well as in materials science, such as in the development of phosphors for lighting and displays.
Conclusion:
The Lanthanoid and Actinoid contractions are a fundamental phenomenon in the chemistry of the f-block elements, resulting from the poor shielding of the nuclear charge by the f-electrons. These contractions have significant effects on the electronic configurations, reactivity, and physical properties of these elements, leading to unique applications in chemistry and materials science. The continued study of the Lanthanoid and Actinoid contractions will undoubtedly lead to further developments in these fields, as well as in our understanding of the fundamental properties of matter.
