The law of mass action is a fundamental principle in chemistry and chemical kinetics that describes the relationship between the concentrations of reactants and products in a chemical reaction at equilibrium. It states that the rate of a chemical reaction is proportional to the product of the concentrations of the reactants, each raised to a power equal to its stoichiometric coefficient in the balanced chemical equation.
In mathematical terms, the law of mass action can be expressed as follows:
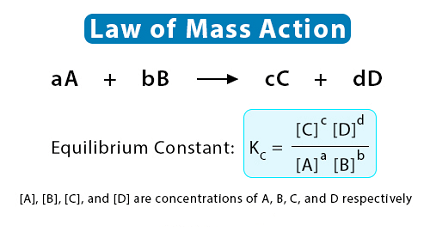
For a chemical reaction aA + bB ⇌ cC + dD, where A, B, C, and D represent the chemical species involved and a, b, c, and d are their stoichiometric coefficients, the equilibrium constant Kc is given by:
Kc = [C]^c [D]^d / [A]^a [B]^b
where [A], [B], [C], and [D] are the molar concentrations of the respective species at equilibrium.
The law of mass action is a powerful tool for predicting the direction and extent of chemical reactions under different conditions. It allows chemists to calculate equilibrium constants and predict the concentrations of reactants and products at equilibrium based on their initial concentrations and the stoichiometry of the reaction.
What is Required Law of mass action
The required conditions for the law of mass action to apply are:
- The chemical reaction must be in a state of equilibrium, meaning that the rates of the forward and reverse reactions are equal.
- The reaction must take place in a homogeneous phase, meaning that all reactants and products are in the same phase (e.g., gas, liquid, or solid).
- The reaction must be a reversible reaction, meaning that it can proceed in both the forward and reverse directions.
- The reaction must follow the law of definite proportions, meaning that the composition of the reactants and products is fixed and can be expressed in terms of their stoichiometric coefficients.
If these conditions are met, then the law of mass action can be used to calculate the equilibrium constant and predict the concentrations of reactants and products at equilibrium. However, it should be noted that the law of mass action is only valid for dilute solutions, and may not hold for reactions in concentrated solutions or in non-ideal conditions.
Who is Required Law of mass action
The law of mass action was first proposed by the French chemist Cato Guldberg and his Norwegian colleague Peter Waage in 1864. They based their theory on observations made during their investigations of the reactions between gases and the kinetics of chemical reactions. Guldberg and Waage proposed that the rates of chemical reactions were determined by the concentrations of the reactants and products, and that the reaction rate would increase with increasing concentrations of the reactants. Their work laid the foundation for modern chemical kinetics and is still widely used today in the study of chemical reactions.
When is Required Law of mass action
The law of mass action is required whenever one wants to study the behavior of a chemical reaction at equilibrium. It is used to predict the direction and extent of a reaction at equilibrium, as well as the equilibrium constant and the concentrations of reactants and products at equilibrium. The law of mass action is applicable in many areas of chemistry, including chemical kinetics, thermodynamics, and electrochemistry. It is used to study a wide variety of chemical reactions, including acid-base reactions, precipitation reactions, complex formation reactions, and many others. Whenever a chemist needs to understand the behavior of a chemical reaction under equilibrium conditions, the law of mass action provides a powerful and fundamental tool for their analysis.
Where is Required Law of mass action
The law of mass action is a fundamental principle of chemical kinetics and equilibrium and is used in many areas of chemistry. It is used to describe the behavior of chemical reactions in a wide range of contexts, including:
- Solution chemistry: The law of mass action is used to study the behavior of chemical reactions in solution, such as acid-base reactions, complex formation reactions, and precipitation reactions.
- Biochemistry: The law of mass action is used to study the behavior of enzymes, which are biological catalysts that facilitate chemical reactions in living organisms.
- Electrochemistry: The law of mass action is used to study the behavior of electrochemical reactions, such as redox reactions and electrodeposition.
- Atmospheric chemistry: The law of mass action is used to study the behavior of chemical reactions in the atmosphere, such as the formation of ozone and other atmospheric pollutants.
Overall, the law of mass action is an important principle in chemistry that is applicable in many areas of research and industry, wherever chemical reactions are studied and manipulated.
How is Required Law of mass action
The law of mass action describes the behavior of chemical reactions at equilibrium and relates the concentrations of reactants and products to the equilibrium constant. The equation for the law of mass action is:
Kc = [C]^c [D]^d / [A]^a [B]^b
where Kc is the equilibrium constant, [A], [B], [C], and [D] are the concentrations of the reactants and products, and a, b, c, and d are the stoichiometric coefficients of the balanced chemical equation.
The law of mass action states that the rate of a chemical reaction is proportional to the product of the concentrations of the reactants, each raised to a power equal to its stoichiometric coefficient in the balanced chemical equation. This means that the rate of the forward reaction is proportional to [A]^a[B]^b, while the rate of the reverse reaction is proportional to [C]^c[D]^d.
When a chemical reaction is at equilibrium, the rates of the forward and reverse reactions are equal. At this point, the concentrations of the reactants and products are constant, and the equilibrium constant can be calculated using the law of mass action. The equilibrium constant gives an indication of the position of the equilibrium, with a high value indicating that the products are favored and a low value indicating that the reactants are favored.
The law of mass action is a powerful tool for predicting the behavior of chemical reactions at equilibrium and is widely used in many areas of chemistry. It allows chemists to calculate the equilibrium constant, predict the concentrations of reactants and products at equilibrium, and optimize reaction conditions to maximize the yield of desired products.
Case Study on Law of mass action
A case study on the law of mass action could involve the study of the Haber-Bosch process, which is used to produce ammonia for use in fertilizers and other industrial applications.
The Haber-Bosch process involves the reaction of nitrogen gas (N2) and hydrogen gas (H2) to form ammonia gas (NH3). The balanced chemical equation for this reaction is:
N2 + 3H2 ⇌ 2NH3
The law of mass action can be used to predict the direction and extent of the reaction at equilibrium, as well as the equilibrium constant and the concentrations of reactants and products at equilibrium.
In the Haber-Bosch process, the reaction is typically carried out at high temperature and pressure, with an iron catalyst. The high temperature and pressure are used to favor the forward reaction, while the iron catalyst is used to increase the rate of the reaction.
The equilibrium constant for the Haber-Bosch process can be calculated using the law of mass action. At equilibrium, the concentrations of nitrogen, hydrogen, and ammonia are constant, and the equilibrium constant can be written as:
Kc = [NH3]^2 / [N2][H2]^3
The value of the equilibrium constant depends on the temperature and pressure at which the reaction is carried out. At standard conditions (25°C and 1 atm), the equilibrium constant for the Haber-Bosch process is approximately 5.7 x 10^-4.
The law of mass action can also be used to predict the concentrations of nitrogen, hydrogen, and ammonia at equilibrium, given the initial concentrations of these species. For example, if the initial concentration of nitrogen is 0.1 M and the initial concentration of hydrogen is 0.3 M, the concentration of ammonia at equilibrium can be calculated using the equilibrium constant and the law of mass action.
Overall, the Haber-Bosch process is a good example of how the law of mass action can be used to study the behavior of chemical reactions at equilibrium and optimize reaction conditions to maximize the yield of desired products.
White paper on Law of mass action
Introduction:
The law of mass action is a fundamental principle of chemical kinetics and equilibrium that describes the behavior of chemical reactions at equilibrium. It relates the concentrations of reactants and products to the equilibrium constant, and can be used to predict the direction and extent of a reaction at equilibrium. In this white paper, we will discuss the law of mass action in detail, including its historical development, its mathematical formulation, and its applications in various fields of chemistry.
Historical Development:
The law of mass action was first proposed by the French chemist Claude Louis Berthollet in 1803. Berthollet observed that the extent of a chemical reaction depended on the concentrations of the reacting species, and proposed that the rate of a chemical reaction was proportional to the product of the concentrations of the reactants. This idea was later refined by other chemists, including Guldberg and Waage in 1864, who developed a mathematical expression for the law of mass action that related the concentrations of reactants and products to the equilibrium constant.
Mathematical Formulation:
The law of mass action is expressed mathematically as:
Kc = [C]^c [D]^d / [A]^a [B]^b
where Kc is the equilibrium constant, [A], [B], [C], and [D] are the concentrations of the reactants and products, and a, b, c, and d are the stoichiometric coefficients of the balanced chemical equation. The equilibrium constant represents the ratio of the concentrations of the products to the concentrations of the reactants at equilibrium, and is a measure of the position of the equilibrium.
Applications:
The law of mass action is used in many areas of chemistry to study the behavior of chemical reactions at equilibrium. It is used in solution chemistry to study acid-base reactions, complex formation reactions, and precipitation reactions. In biochemistry, the law of mass action is used to study the behavior of enzymes and other biological molecules. In electrochemistry, the law of mass action is used to study the behavior of redox reactions and electrodeposition. In atmospheric chemistry, the law of mass action is used to study the behavior of chemical reactions in the atmosphere.
The law of mass action is also used to optimize reaction conditions and maximize the yield of desired products. For example, in the production of ammonia by the Haber-Bosch process, the law of mass action is used to predict the equilibrium constant and the concentrations of nitrogen, hydrogen, and ammonia at equilibrium, and to optimize the reaction conditions to maximize the yield of ammonia.
Conclusion:
In conclusion, the law of mass action is a fundamental principle of chemical kinetics and equilibrium that describes the behavior of chemical reactions at equilibrium. It is a powerful tool for predicting the direction and extent of a reaction at equilibrium, and for optimizing reaction conditions to maximize the yield of desired products. The law of mass action has applications in many areas of chemistry, and has played a crucial role in the development of modern chemistry and chemical engineering.
