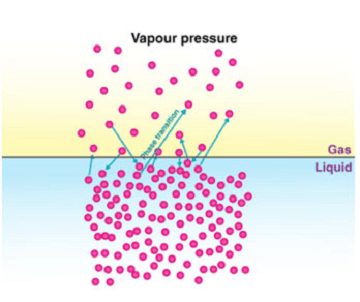
Vapor pressure is the pressure that a vapor exerts when it is in equilibrium with its condensed phase (liquid or solid) at a given temperature. In other words, it is the pressure exerted by the molecules of a liquid or solid when they evaporate and form a vapor above the surface of the liquid or solid.
The vapor pressure of a liquid depends on the temperature, the intermolecular forces between the molecules of the liquid, and the surface area of the liquid exposed to the air. Generally, as the temperature increases, the vapor pressure of a liquid also increases because the molecules have more energy and can more easily overcome the intermolecular forces holding them in the liquid phase.
The relationship between vapor pressure and temperature can be described by the Clausius-Clapeyron equation, which states that the natural logarithm of the vapor pressure of a liquid is proportional to the reciprocal of the absolute temperature:
ln(P) = -ΔHvap/R(1/T) + C
where P is the vapor pressure, ΔHvap is the enthalpy of vaporization, R is the gas constant, T is the absolute temperature, and C is a constant.
The vapor pressure of a liquid is important in a variety of applications, including in the design of chemical processes, the calculation of vapor-liquid equilibrium, and the study of atmospheric science.
What is Required Liquids: Vapour pressure
The vapor pressure of a liquid is required in various fields of science and engineering. It is a fundamental property of liquids and is necessary for many calculations and design considerations. Here are some examples:
- Chemical Engineering: Vapor pressure is an important parameter in the design of distillation columns used in separating components of a mixture. The vapor pressure of each component is used to calculate the composition of the vapor and liquid phases at each stage of the distillation column.
- Atmospheric Science: The vapor pressure of a liquid is important in understanding the behavior of atmospheric gases and their interactions with the environment. For example, the vapor pressure of water is important in determining the relative humidity of the air.
- Materials Science: The vapor pressure of a liquid is important in determining the stability and performance of materials. For example, the vapor pressure of solvents used in coatings can affect the drying time and quality of the coating.
- Biomedical Science: The vapor pressure of liquids is important in the design and operation of medical devices such as inhalers and vaporizers. The vapor pressure of a drug determines the rate and amount of the drug delivered to the patient.
Overall, the vapor pressure of liquids is an important property that is required in various fields of science and engineering, and it is used in many different applications to understand and design various processes and devices.
Who is Required Liquids: Vapour pressure
The concept of vapor pressure and the knowledge of the vapor pressure of liquids is required by many professionals in different fields, including:
- Chemists: Chemists need to understand the vapor pressure of liquids to design and optimize chemical reactions, to determine the boiling points and distillation ranges of liquids, and to calculate vapor-liquid equilibria.
- Chemical engineers: Chemical engineers use the vapor pressure of liquids to design and optimize processes for separating, purifying, and refining chemicals, such as distillation, evaporation, and drying processes.
- Atmospheric scientists: Atmospheric scientists study the behavior of gases and particles in the atmosphere, including the evaporation and condensation of liquids. They use the vapor pressure of liquids to understand the behavior of water and other volatile compounds in the atmosphere, which is important for predicting weather patterns and air quality.
- Materials scientists: Materials scientists use the vapor pressure of liquids to understand and design materials that are stable in a range of environments. They also use the vapor pressure of solvents and other liquids to optimize coatings, adhesives, and other materials.
- Biomedical scientists and pharmacists: Biomedical scientists and pharmacists use the vapor pressure of liquids to design and optimize medical devices, such as inhalers and vaporizers, and to understand the pharmacokinetics of drugs.
Overall, anyone who works with liquids, whether in a scientific or industrial context, may need to understand the concept of vapor pressure and the vapor pressure of specific liquids in order to make informed decisions and optimize processes.
When is Required Liquids: Vapour pressure
The knowledge of the vapor pressure of liquids is required in a wide range of situations, including:
- Distillation: The vapor pressure of liquids is crucial in designing and optimizing distillation processes, which are used to separate and purify different components of a mixture based on their boiling points.
- Evaporation: The vapor pressure of liquids also plays a key role in the design of evaporators, which are used to remove water or other solvents from a solution or suspension.
- Atmospheric Science: Understanding the vapor pressure of liquids is important in atmospheric science to understand the behavior of gases and particles in the atmosphere, including the formation and behavior of clouds and aerosols.
- Materials Science: The vapor pressure of liquids is important in the design and testing of materials, including coatings, adhesives, and films, where it can affect the stability and performance of the material.
- Medical Devices: The vapor pressure of liquids is important in the design of medical devices that use vaporization or nebulization to deliver drugs to the body, such as inhalers, vaporizers, and nebulizers.
- Chemical Reactions: The vapor pressure of liquids can affect the rates and yields of chemical reactions, especially those involving volatile or gaseous products.
Overall, the knowledge of the vapor pressure of liquids is required in many different fields, from chemical engineering and materials science to atmospheric science and medical devices.
Where is Required Liquids: Vapour pressure
The knowledge of the vapor pressure of liquids is required in many different fields and industries, including:
- Chemical industry: The chemical industry uses the vapor pressure of liquids to design and optimize various processes, including distillation, extraction, and evaporation, for the production of chemicals and materials.
- Petroleum industry: The petroleum industry uses the vapor pressure of liquids to measure the volatility of fuels and other petroleum products, which affects their stability and flammability.
- Food and beverage industry: The food and beverage industry uses the vapor pressure of liquids to design and optimize processes such as drying, concentration, and extraction of flavors and fragrances.
- Pharmaceutical industry: The pharmaceutical industry uses the vapor pressure of liquids to design and optimize processes for drug delivery, such as inhalation, and to measure the stability and shelf life of drugs.
- Atmospheric science: Atmospheric scientists use the vapor pressure of liquids to understand the behavior of gases and particles in the atmosphere, including the formation of clouds, precipitation, and air pollution.
- Materials science: The materials science industry uses the vapor pressure of liquids to design and optimize various materials, including coatings, adhesives, and films, for various applications such as protective coatings, optical films, and electronics.
Overall, the knowledge of the vapor pressure of liquids is required in many different fields and industries, where it is used to design and optimize various processes and products.
How is Required Liquids: Vapour pressure
The vapor pressure of liquids can be determined experimentally or calculated theoretically using various methods. Here are some of the ways that the vapor pressure of liquids can be determined:
- Boiling point: The boiling point of a liquid is the temperature at which its vapor pressure equals the atmospheric pressure. By measuring the boiling point at a given pressure, the vapor pressure of the liquid can be calculated using standard thermodynamic equations.
- Antoine equation: The Antoine equation is an empirical equation that relates the vapor pressure of a pure substance to its temperature. It can be used to calculate the vapor pressure of liquids over a wide range of temperatures.
- Clausius-Clapeyron equation: The Clausius-Clapeyron equation is a thermodynamic equation that relates the vapor pressure of a substance to its enthalpy of vaporization and temperature. It can be used to calculate the vapor pressure of liquids at different temperatures and pressures.
- Knudsen effusion method: The Knudsen effusion method is an experimental method that measures the vapor pressure of a substance by measuring the rate at which molecules escape from a small hole in a container containing the liquid.
- Dynamic vapor sorption: Dynamic vapor sorption is a method that measures the uptake and release of vapor by a sample using a controlled humidity environment. By measuring the amount of vapor that is sorbed by the sample, the vapor pressure of the liquid can be determined.
Overall, the vapor pressure of liquids can be determined experimentally or calculated theoretically using various methods, depending on the specific application and the accuracy required.
Case Study on Liquids: Vapour pressure
One example of the importance of understanding the vapor pressure of liquids can be seen in the production of pharmaceuticals. The vapor pressure of a liquid drug can affect its stability, efficacy, and safety, as well as its route of administration.
For instance, many inhalation drugs are formulated as liquids that are vaporized and inhaled as a mist or aerosol. The vapor pressure of these liquids determines their ability to vaporize at room temperature and pressure and to produce an aerosol that can be inhaled effectively. A liquid with a high vapor pressure will be more easily vaporized and can produce a larger amount of aerosol particles, leading to better drug delivery to the lungs.
On the other hand, a liquid with a low vapor pressure may be more stable and have a longer shelf life, but it may require higher temperatures or pressures to vaporize and may produce a smaller amount of aerosol particles, leading to poor drug delivery.
Furthermore, the vapor pressure of a liquid can also affect its safety and potential for abuse. For example, liquid opioids such as fentanyl have high vapor pressures, which can increase the risk of accidental exposure and overdose through inhalation or skin contact.
Therefore, understanding the vapor pressure of liquids is crucial in the formulation and production of pharmaceuticals, particularly those delivered by inhalation, and can affect the drug’s efficacy, stability, safety, and abuse potential.
White paper on Liquids: Vapour pressure
Introduction:
Liquids are a ubiquitous form of matter that are essential for many industrial, scientific, and everyday applications. The vapor pressure of liquids is an important physical property that determines their volatility, evaporation rate, and boiling point. In this white paper, we will discuss the importance of understanding the vapor pressure of liquids, its measurement methods, and its applications in different fields.
The Importance of Vapor Pressure of Liquids:
The vapor pressure of a liquid is the pressure exerted by its molecules in the gas phase above its surface in equilibrium with the liquid phase at a given temperature. It is an important property that influences the behavior of liquids and their interactions with the environment. Understanding the vapor pressure of liquids is critical for many applications, including:
- Process design and optimization: The vapor pressure of liquids is essential for designing and optimizing processes such as distillation, extraction, and drying in the chemical, food, and pharmaceutical industries. By knowing the vapor pressure of a liquid, one can determine the temperature and pressure conditions required to separate or extract the desired components efficiently.
- Safety and environmental impact: The vapor pressure of liquids can affect their safety and environmental impact. For example, liquids with high vapor pressure, such as volatile organic compounds (VOCs), can easily evaporate into the air and cause air pollution or health hazards. Understanding the vapor pressure of these liquids can help to prevent or mitigate their negative impact on the environment and human health.
- Material properties: The vapor pressure of liquids can affect the properties of materials such as coatings, films, and adhesives. For example, the vapor pressure of the solvent in a coating or adhesive can affect its curing rate, adhesion, and stability. Understanding the vapor pressure of liquids can help to optimize the properties of materials for different applications.
Measurement Methods of Vapor Pressure of Liquids:
There are several experimental and theoretical methods for measuring the vapor pressure of liquids. Some of the most common methods are:
- Boiling point method: The boiling point of a liquid is the temperature at which its vapor pressure equals the atmospheric pressure. By measuring the boiling point of a liquid at a given pressure, its vapor pressure can be calculated using thermodynamic equations.
- Antoine equation: The Antoine equation is an empirical equation that relates the vapor pressure of a pure substance to its temperature. It can be used to calculate the vapor pressure of liquids over a wide range of temperatures.
- Knudsen effusion method: The Knudsen effusion method measures the vapor pressure of a liquid by measuring the rate at which molecules escape from a small hole in a container containing the liquid.
- Dynamic vapor sorption: Dynamic vapor sorption is a method that measures the uptake and release of vapor by a sample using a controlled humidity environment. By measuring the amount of vapor that is sorbed by the sample, the vapor pressure of the liquid can be determined.
Applications of Vapor Pressure of Liquids:
The vapor pressure of liquids has many applications in different fields, including:
- Chemical industry: The chemical industry uses the vapor pressure of liquids to design and optimize various processes such as distillation, extraction, and evaporation for the production of chemicals and materials.
- Petroleum industry: The petroleum industry uses the vapor pressure of liquids to measure the volatility of fuels and other petroleum products, which affects their stability and flammability.
- Food and beverage industry: The food and beverage industry uses the vapor pressure of liquids to design and optimize processes such as drying, concentration, and extraction of flavors and fragrances.
- Pharmaceutical industry: The pharmaceutical industry uses the vapor pressure of liquids to design and optimize processes for drug delivery, such as inhalation, and to measure the stability and shelf life of drugs.
Conclusion:
In conclusion, understanding the vapor pressure of liquids is essential for various applications in different fields, including process design and optimization, safety and environmental impact, and material properties. There are several methods for measuring the vapor pressure of liquids, including the boiling point method, Antoine equation, Knudsen effusion method, and dynamic vapor sorption. The vapor pressure of liquids has significant applications in the chemical, petroleum, food and beverage, and pharmaceutical industries. Therefore, a better understanding of this physical property can lead to the development of more efficient and sustainable processes and products.
