Metal carbonyls are compounds that consist of metal atoms coordinated to one or more carbon monoxide (CO) molecules. They are important in organometallic chemistry and have many industrial applications, particularly as catalysts in chemical reactions.
The most well-known metal carbonyl is probably iron pentacarbonyl (Fe(CO)5), which is a colorless, volatile liquid that is used as a precursor to the production of high-purity iron and steel. Other metal carbonyls include nickel tetracarbonyl (Ni(CO)4), which is also a colorless liquid, and tungsten hexacarbonyl (W(CO)6), which is a yellow solid.
Metal carbonyls have interesting properties, such as high volatility and low melting points, and they can form clusters of multiple metal atoms coordinated to CO ligands. They are also known to undergo ligand substitution reactions, where other ligands can replace the CO ligands, resulting in different metal complexes with different properties.
While metal carbonyls have many useful applications, they can also be toxic and pose health risks if not handled properly. For example, exposure to nickel carbonyl (Ni(CO)4) can be fatal, as it can quickly enter the bloodstream and cause severe respiratory and neurological symptoms.
What is Required Coordination Compounds Metal carbonyls
Coordination compounds, also known as complex compounds, are molecules or ions consisting of a central metal ion or atom coordinated to one or more ligands. Metal carbonyls are a type of coordination compound that consist of metal atoms coordinated to one or more carbon monoxide (CO) molecules.
Metal carbonyls are important in coordination chemistry and have many industrial applications, particularly as catalysts in chemical reactions. They have interesting properties, such as high volatility and low melting points, and they can form clusters of multiple metal atoms coordinated to CO ligands.
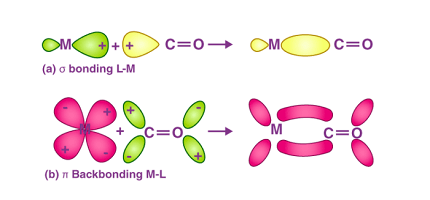
In terms of their coordination chemistry, metal carbonyls are classified as “sigma” complexes because the CO ligands are bonded to the metal through the sigma bonds between the metal and the carbon monoxide molecule. The CO ligands can also form pi bonds with the metal atom, resulting in pi complexes.
Metal carbonyls have been extensively studied and have contributed significantly to our understanding of coordination chemistry and the bonding between metal atoms and ligands. They also have practical applications in areas such as catalysis, materials science, and electronics.
When is Required Coordination Compounds Metal carbonyls
Coordination compounds, including metal carbonyls, are required in a variety of fields, ranging from academic research to industrial applications. Here are a few examples:
- Catalysis: Metal carbonyls are widely used as catalysts in a variety of chemical reactions, including hydrogenation, hydroformylation, and carbonylation reactions. For example, the reaction of carbon monoxide and hydrogen to produce methanol is catalyzed by metal carbonyls such as rhodium carbonyl.
- Materials science: Metal carbonyls have been used to synthesize a variety of materials, including nanoparticles, thin films, and metal-organic frameworks. These materials have a range of potential applications, such as in electronics, sensors, and catalysis.
- Medicinal chemistry: Metal carbonyls have shown promise as anticancer agents and have been investigated for their potential use in cancer therapy. For example, osmium carbonyl complexes have been shown to inhibit the growth of cancer cells.
- Basic research: Metal carbonyls have played an important role in the development of coordination chemistry as a field of study. They have been used to study the bonding between metal atoms and ligands and to investigate the properties of metal clusters and organometallic compounds.
In summary, metal carbonyls and other coordination compounds are required in a variety of fields, from catalysis to materials science to basic research. Their unique properties and versatile chemistry make them important tools for scientists and engineers in a range of disciplines.
Where is Required Coordination Compounds Metal carbonyls
Metal carbonyls and other coordination compounds are found in a variety of places, including academic and industrial laboratories, as well as in commercial products. Here are a few examples:
- Research laboratories: Metal carbonyls are commonly used in academic and industrial research laboratories to study the chemistry of coordination compounds and to develop new materials and catalysts.
- Chemical manufacturing: Metal carbonyls are used as catalysts in a range of chemical reactions, particularly in the production of organic compounds such as plastics, polymers, and pharmaceuticals.
- Electronics industry: Metal carbonyls have potential applications in the electronics industry, particularly as precursors for the synthesis of thin films and nanomaterials used in electronic devices.
- Medical industry: Metal carbonyls have shown potential as anticancer agents, and are being investigated for their use in cancer therapy.
- Consumer products: Metal carbonyls are used in the production of a range of consumer products, such as coatings, adhesives, and inks.
Overall, metal carbonyls and other coordination compounds are used in a wide variety of industries and applications. Their unique properties and versatile chemistry make them important tools for scientists and engineers in many different fields.
How is Required Coordination Compounds Metal carbonyls
The synthesis of metal carbonyls and other coordination compounds typically involves the reaction of a metal salt or metal complex with a ligand. Here are the general steps involved in synthesizing metal carbonyls:
- Preparation of the metal precursor: The metal precursor can be a metal salt or a metal complex. In some cases, the metal precursor is first reduced to the metal atom before coordination with the ligand.
- Coordination of the ligand: The ligand, in this case carbon monoxide, is added to the metal precursor. The reaction can occur under various conditions, such as in the presence of a catalyst or under high pressure.
- Purification: The resulting metal carbonyl complex is typically purified through various techniques, such as distillation, chromatography, or recrystallization.
The specific conditions used to synthesize metal carbonyls depend on the type of metal and ligand being used, as well as the desired properties of the resulting complex. For example, some metal carbonyls are synthesized under high pressure, while others are synthesized under vacuum. The purity of the starting materials, reaction temperature, and choice of solvent can also affect the synthesis process.
Overall, the synthesis of metal carbonyls and other coordination compounds requires careful control of reaction conditions to ensure the desired product is obtained in high yield and purity.
Nomenclature of Coordination Compounds Metal carbonyls
The nomenclature of metal carbonyls and other coordination compounds follows the rules established by the International Union of Pure and Applied Chemistry (IUPAC). The name of a coordination compound generally consists of three parts:
- The name of the ligands, listed in alphabetical order, followed by the central metal atom or ion. In the case of metal carbonyls, the ligand name is “carbonyl,” and the metal atom or ion is named according to its charge and the standard naming conventions for that element.
- The oxidation state of the metal ion or atom, written in Roman numerals in parentheses.
- The name of the counterion or other ions in the compound, if present.
Here are a few examples of the nomenclature of metal carbonyls:
- Iron pentacarbonyl: This compound consists of iron coordinated to five carbon monoxide molecules. The name is derived from “penta,” meaning five, and “carbonyl.” The oxidation state of iron is zero, since each CO molecule donates two electrons to the metal. Therefore, the compound is simply named “iron pentacarbonyl.”
- Nickel tetracarbonyl: This compound consists of nickel coordinated to four carbon monoxide molecules. The name is derived from “tetra,” meaning four, and “carbonyl.” The oxidation state of nickel is zero, since each CO molecule donates two electrons to the metal. Therefore, the compound is simply named “nickel tetracarbonyl.”
- Molybdenum hexacarbonyl: This compound consists of molybdenum coordinated to six carbon monoxide molecules. The name is derived from “hexa,” meaning six, and “carbonyl.” The oxidation state of molybdenum is zero, since each CO molecule donates two electrons to the metal. Therefore, the compound is simply named “molybdenum hexacarbonyl.”
The nomenclature of coordination compounds can become more complex when multiple ligands are present or when the central metal atom or ion has multiple oxidation states. However, the basic principles outlined above still apply.
Case Study on Coordination Compounds Metal carbonyls
One interesting case study on the application of metal carbonyls is the use of iron pentacarbonyl in the production of magnetic iron oxide nanoparticles.
Iron oxide nanoparticles are commonly used in a range of applications, including drug delivery, imaging, and magnetic separation. However, the synthesis of these nanoparticles can be challenging, particularly in terms of controlling particle size and morphology.
Iron pentacarbonyl is a common precursor for the synthesis of iron oxide nanoparticles. The precursor is first heated to a high temperature, causing the CO ligands to dissociate and leaving behind iron atoms. These iron atoms can then react with oxygen or water to form iron oxide nanoparticles.
By controlling the reaction conditions, such as temperature, pressure, and reaction time, it is possible to produce iron oxide nanoparticles with precise size and morphology. In addition, the use of iron pentacarbonyl as a precursor can result in nanoparticles with high purity and low levels of impurities.
One advantage of using metal carbonyls as precursors for nanoparticle synthesis is that the reaction can occur at relatively low temperatures, reducing the risk of thermal decomposition or oxidation. In addition, the use of metal carbonyls can result in more uniform nanoparticle size and morphology compared to other methods.
Overall, the use of metal carbonyls such as iron pentacarbonyl has the potential to improve the synthesis of a range of nanoparticle materials, including iron oxide nanoparticles, with potential applications in areas such as biomedicine, environmental remediation, and energy storage.
White paper on Coordination Compounds Metal carbonyls
Introduction:
Coordination compounds are complexes that contain a central metal ion or atom that is coordinated to one or more ligands. These ligands can be atoms, ions, or molecules that donate one or more pairs of electrons to the metal ion. One class of coordination compounds that has received significant attention is metal carbonyls, which consist of a metal atom or ion coordinated to one or more carbon monoxide (CO) molecules. In this white paper, we will discuss the properties, synthesis, and applications of metal carbonyls.
Properties:
Metal carbonyls have unique properties that make them useful in a range of applications. One of the most striking features of metal carbonyls is their ability to bind to carbon monoxide in a highly specific manner. This selectivity is due to the fact that carbon monoxide is a strong sigma donor and pi acceptor, making it an ideal ligand for metals with empty d-orbitals. In addition, metal carbonyls are often volatile and can be used as precursors for the synthesis of nanoparticles and other materials.
Synthesis:
The synthesis of metal carbonyls typically involves the reaction of a metal precursor with carbon monoxide in the presence of a catalyst or under high pressure. The metal precursor can be a metal salt or metal complex, and the reaction conditions can be tuned to control the size and morphology of the resulting metal carbonyl complex. Common techniques for synthesizing metal carbonyls include thermal decomposition, photochemical reactions, and electrochemical methods.
Applications:
Metal carbonyls have a wide range of applications, from catalysis to materials science. One important application of metal carbonyls is their use as precursors for the synthesis of metal nanoparticles. For example, iron pentacarbonyl is commonly used as a precursor for the synthesis of iron oxide nanoparticles, which have applications in biomedicine and environmental remediation. Metal carbonyls can also be used as catalysts for a range of reactions, including hydrogenation and oxidation reactions. In addition, metal carbonyls have been used as molecular probes in a variety of imaging techniques, including infrared spectroscopy and magnetic resonance imaging.
Conclusion:
Metal carbonyls are an important class of coordination compounds that exhibit unique properties and have a wide range of applications. The selectivity of metal carbonyls for carbon monoxide, as well as their volatility and ease of synthesis, make them useful in the production of nanoparticles and other materials. In addition, metal carbonyls can be used as catalysts for a range of reactions and as molecular probes in imaging techniques. Overall, the study of metal carbonyls has the potential to improve our understanding of coordination chemistry and advance a range of important applications in fields such as materials science, biomedicine, and environmental remediation.