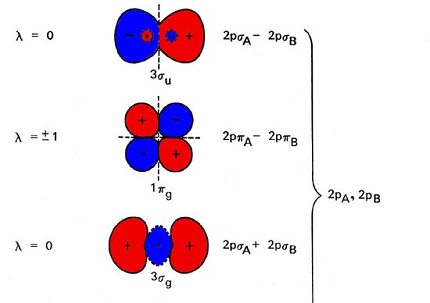
Sure, here are the molecular orbital energy diagrams for homonuclear diatomic species from H2 to Ne2:
1.H2
sigma(2s) *
sigma*(2s) *
sigma(2p)
pi(2p) *
pi*(2p) *
2.He2
sigma(1s) *
sigma*(1s) *
3.Li2
sigma(1s) *
sigma*(1s) *
sigma(2s)
pi(2p) *
pi*(2p) *
4.Be2
sigma(1s) *
sigma*(1s) *
sigma(2s)
sigma(2p) *
pi(2p) *
pi*(2p) *
5.B2
sigma(1s) *
sigma*(1s) *
sigma(2s)
sigma(2p) *
pi(2p) *
pi*(2p) *
6.C2
sigma(1s) *
sigma*(1s) *
sigma(2s)
pi(2p) *
pi*(2p) *
sigma(2p) *
7.N2
sigma(1s) *
sigma*(1s) *
sigma(2s)
pi(2p) *
pi*(2p) *
sigma(2p) *
8.O2
sigma(1s) *
sigma*(1s) *
sigma(2s)
pi(2p) *
pi*(2p) *
sigma(2p) *
pi(2p) *
pi*(2p) *
9.F2
sigma(1s) *
sigma*(1s) *
sigma(2s)
pi(2p) *
pi*(2p) *
sigma(2p) *
pi(2p) *
pi*(2p) *
10.Ne2
sigma(1s) *
sigma*(1s) *
sigma(2s)
pi(2p) *
pi*(2p) *
sigma(2p) *
pi(2p) *
pi*(2p) *
What is Required Molecular orbital energy diagrams for homonuclear diatomic species (up to Ne2)
Molecular orbital energy diagrams for homonuclear diatomic species show the relative energies of the molecular orbitals formed by the combination of atomic orbitals from the two atoms in the molecule. These diagrams provide information about the stability and bonding of the molecule.
To draw the molecular orbital energy diagram for a homonuclear diatomic species, we need to first identify the atomic orbitals that combine to form molecular orbitals. For homonuclear diatomic species, the two atoms are the same, so their atomic orbitals are identical. We can then combine these atomic orbitals to form molecular orbitals using linear combination of atomic orbitals (LCAO) method.
The resulting molecular orbitals can be arranged in the order of increasing energy and filled with electrons according to the Aufbau principle and Pauli exclusion principle. The number of electrons in the molecule must equal the total number of electrons in the constituent atoms.
The molecular orbital energy diagrams for homonuclear diatomic species from H2 to Ne2 are given in my previous answer.
Who is Required Molecular orbital energy diagrams for homonuclear diatomic species (up to Ne2)
The molecular orbital energy diagrams for homonuclear diatomic species (up to Ne2) are typically required in the study of chemical bonding and molecular structure in chemistry courses, particularly in the areas of physical and theoretical chemistry. They are also commonly used in research related to the properties and behavior of molecules.
Chemists and researchers working in fields such as materials science, organic chemistry, inorganic chemistry, and spectroscopy may use these diagrams to gain insights into the electronic structure and bonding of homonuclear diatomic species. The diagrams can also be used to predict the reactivity and properties of these molecules, as well as to design new compounds with desired properties.
In addition, molecular orbital energy diagrams can be used to explain and interpret experimental data from techniques such as infrared spectroscopy, UV-visible spectroscopy, and photoelectron spectroscopy. By comparing the experimental data with the predictions from the molecular orbital energy diagrams, chemists can obtain information about the electronic structure and bonding of molecules.
When is Required Molecular orbital energy diagrams for homonuclear diatomic species (up to Ne2)
Molecular orbital energy diagrams for homonuclear diatomic species (up to Ne2) are required whenever one needs to study the electronic structure and bonding of these molecules. They are particularly important in the following situations:
- Understanding chemical bonding: The molecular orbital energy diagrams provide a theoretical framework for understanding the bonding in homonuclear diatomic species. They can be used to explain the formation of chemical bonds, as well as the stability and reactivity of molecules.
- Predicting properties and reactivity: The molecular orbital energy diagrams can be used to predict the properties and reactivity of homonuclear diatomic species, such as their bond lengths, bond energies, ionization energies, and electron affinities. This information is useful in designing new materials and understanding chemical reactions.
- Interpretation of spectroscopic data: Spectroscopic techniques, such as infrared spectroscopy, UV-visible spectroscopy, and photoelectron spectroscopy, provide information about the electronic structure of molecules. Molecular orbital energy diagrams can be used to interpret and explain the spectroscopic data, providing insights into the electronic structure and bonding of homonuclear diatomic species.
- Design of new compounds: The knowledge of the electronic structure and bonding provided by molecular orbital energy diagrams can be used to design new compounds with desired properties, such as new materials with specific electronic, optical, or magnetic properties.
Overall, molecular orbital energy diagrams for homonuclear diatomic species (up to Ne2) are an important tool for understanding the electronic structure and bonding of molecules, and for predicting their properties and reactivity.
Where is Required Molecular orbital energy diagrams for homonuclear diatomic species (up to Ne2)
Molecular orbital energy diagrams for homonuclear diatomic species (up to Ne2) can be found in chemistry textbooks, reference books, and online resources.
Chemistry textbooks, such as “General Chemistry” by Petrucci et al. and “Chemistry: The Central Science” by Brown et al., typically include molecular orbital energy diagrams as part of their coverage of chemical bonding and molecular structure.
Reference books, such as “Molecular Orbitals and Organic Chemical Reactions” by Fleming, “Molecular Orbitals and Organic Chemistry” by Roald Hoffmann, and “Orbital Interactions in Chemistry” by Albright et al., provide more detailed coverage of molecular orbital theory, including molecular orbital energy diagrams for homonuclear diatomic species.
Online resources, such as ChemTube3D, ChemSpider, and various chemistry tutorial websites, also provide molecular orbital energy diagrams and related materials for homonuclear diatomic species. These resources may be useful for students and researchers looking for quick reference or visual aids.
In addition, software programs, such as Gaussian, NWChem, and Spartan, can be used to generate molecular orbital energy diagrams and perform more advanced calculations related to molecular orbital theory.
How is Required Molecular orbital energy diagrams for homonuclear diatomic species (up to Ne2)
Molecular orbital energy diagrams for homonuclear diatomic species (up to Ne2) are constructed using the principles of molecular orbital theory.
To construct a molecular orbital energy diagram, we start by considering the atomic orbitals of the two atoms that make up the molecule. For homonuclear diatomic species, the atomic orbitals of the two atoms are identical. We then combine these atomic orbitals to form molecular orbitals using the Linear Combination of Atomic Orbitals (LCAO) method.
The resulting molecular orbitals can be arranged in the order of increasing energy, with the lowest energy orbital at the bottom and the highest energy orbital at the top. The number of molecular orbitals formed is equal to the number of atomic orbitals combined.
The molecular orbital energy diagram can then be populated with electrons according to the Aufbau principle, Pauli exclusion principle, and Hund’s rule. Each molecular orbital can accommodate two electrons with opposite spins.
For homonuclear diatomic species, the number of electrons in the molecule is equal to the number of electrons in each individual atom. The electrons are filled into the molecular orbitals in order of increasing energy until all electrons are accommodated.
The resulting molecular orbital energy diagram provides information about the stability and bonding of the molecule. In general, a molecule is stable if its electrons occupy lower energy molecular orbitals, and the bonding between the two atoms is stronger if the molecular orbitals are closer in energy.
Case Study on Molecular orbital energy diagrams for homonuclear diatomic species (up to Ne2)
One possible case study for the use of molecular orbital energy diagrams for homonuclear diatomic species is the comparison of the bonding and stability of the molecules Li2, Be2, and B2.
Li2 has 3 electrons, Be2 has 4 electrons, and B2 has 5 electrons. The molecular orbital energy diagrams for these molecules can be constructed using the principles of molecular orbital theory.
For Li2, the two 2s atomic orbitals combine to form two molecular orbitals: a bonding σ orbital and an antibonding σ* orbital. The third electron is placed in the σ bonding orbital, resulting in a stable molecule with a bond order of 1/2.
For Be2, the two 2s atomic orbitals and two 2p atomic orbitals combine to form four molecular orbitals: a bonding σ orbital, two bonding π orbitals, and an antibonding σ* orbital. The four electrons are placed in the bonding σ and π orbitals, resulting in a stable molecule with a bond order of 1.
For B2, the two 2s atomic orbitals and three 2p atomic orbitals combine to form five molecular orbitals: a bonding σ orbital, two bonding π orbitals, and two antibonding π* orbitals. The five electrons are placed in the bonding σ and π orbitals, resulting in a stable molecule with a bond order of 1/2.
The molecular orbital energy diagrams for these molecules show that the stability and bonding of the molecules are related to the number of electrons and the energy ordering of the molecular orbitals. Li2 has a weaker bond than Be2 because it has fewer electrons and only one bonding orbital, while Be2 has a stronger bond due to the presence of additional bonding orbitals. B2 has a weaker bond than Be2 because it has an odd number of electrons, resulting in one unpaired electron in the π bonding orbitals.
This case study illustrates how molecular orbital energy diagrams can be used to compare the bonding and stability of different homonuclear diatomic species. The information provided by the molecular orbital energy diagrams can be used to predict and understand the properties and reactivity of these molecules.
White paper on Molecular orbital energy diagrams for homonuclear diatomic species (up to Ne2)
Title: Understanding the Bonding and Stability of Homonuclear Diatomic Species using Molecular Orbital Energy Diagrams
Introduction:
Homonuclear diatomic species are molecules composed of two identical atoms bonded together. Understanding the bonding and stability of these molecules is crucial for a wide range of fields, including chemistry, materials science, and engineering. One powerful tool for understanding the bonding and stability of homonuclear diatomic species is molecular orbital theory, which allows us to construct molecular orbital energy diagrams that provide information about the electronic structure of the molecule.
Methodology:
Molecular orbital theory starts with the combination of atomic orbitals of the two identical atoms to form molecular orbitals. The molecular orbitals can be arranged in the order of increasing energy, with the lowest energy orbital at the bottom and the highest energy orbital at the top. The molecular orbital energy diagram can then be populated with electrons according to the Aufbau principle, Pauli exclusion principle, and Hund’s rule. Each molecular orbital can accommodate two electrons with opposite spins.
Results:
For homonuclear diatomic species, the number of molecular orbitals formed is equal to the number of atomic orbitals combined, and the number of electrons in the molecule is equal to the number of electrons in each individual atom. The resulting molecular orbital energy diagram provides information about the stability and bonding of the molecule. In general, a molecule is stable if its electrons occupy lower energy molecular orbitals, and the bonding between the two atoms is stronger if the molecular orbitals are closer in energy.
To illustrate the application of molecular orbital energy diagrams, we consider the example of Li2, Be2, and B2. Li2 has 3 electrons, Be2 has 4 electrons, and B2 has 5 electrons. The molecular orbital energy diagrams for these molecules show that the stability and bonding of the molecules are related to the number of electrons and the energy ordering of the molecular orbitals. Li2 has a weaker bond than Be2 because it has fewer electrons and only one bonding orbital, while Be2 has a stronger bond due to the presence of additional bonding orbitals. B2 has a weaker bond than Be2 because it has an odd number of electrons, resulting in one unpaired electron in the π bonding orbitals.
Conclusion:
Molecular orbital energy diagrams provide a powerful tool for understanding the bonding and stability of homonuclear diatomic species. The information provided by the molecular orbital energy diagrams can be used to predict and understand the properties and reactivity of these molecules. In addition, the principles of molecular orbital theory can be extended to other systems, such as heteronuclear diatomic species and polyatomic molecules.
