Atoms and Nuclei
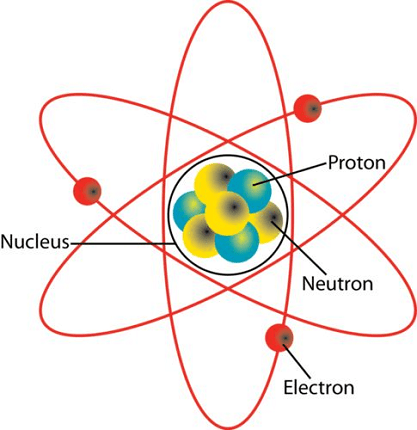
Atoms and nuclei are fundamental concepts in the field of physics that describe the structure and behavior of matter at the atomic and subatomic levels. Here’s a detailed explanation of atoms and nuclei:
Atoms: An atom is the smallest unit of matter that retains the chemical properties of an element. It consists of a positively charged nucleus at the center and negatively charged electrons orbiting around it. The nucleus contains protons and neutrons, collectively known as nucleons. The number of protons determines the atomic number of an atom, which defines its unique identity as an element. Electrons, which have a much smaller mass than protons and neutrons, occupy discrete energy levels or orbitals around the nucleus.
Key concepts related to atoms include:
- Atomic Structure: The arrangement of protons, neutrons, and electrons within an atom.
- Atomic Models: Various models, such as the Thomson, Rutherford, and Bohr models, have been proposed to explain atomic structure.
- Quantum Mechanics: Describes the behavior of electrons as both particles and waves, and it provides a more accurate description of atomic structure than classical physics.
- Electron Configurations: The distribution of electrons in different energy levels or orbitals around the nucleus.
- Atomic Spectra: The characteristic patterns of light emitted or absorbed by atoms, which provide information about their energy levels and electron transitions.
- Chemical Bonding: The interaction of atoms through the sharing or transfer of electrons, leading to the formation of molecules and compounds.
Nuclei: The nucleus is the central part of an atom that contains protons and neutrons tightly bound together by the strong nuclear force. It is responsible for the majority of an atom’s mass. The number of protons in the nucleus determines the atomic number, while the total number of protons and neutrons defines the mass number of an atom.
Key concepts related to nuclei include:
- Nuclear Structure: The organization of protons and neutrons within the nucleus.
- Nuclear Forces: The strong nuclear force that binds protons and neutrons together, overcoming the electrostatic repulsion between protons.
- Isotopes and Isobars: Atoms of the same element with different numbers of neutrons are called isotopes. Isobars are atoms with the same mass number but different atomic numbers.
- Radioactivity: The spontaneous decay of unstable nuclei, resulting in the emission of particles or electromagnetic radiation.
- Radioactive Decay: The process by which unstable nuclei transform into more stable configurations, involving alpha decay (emission of alpha particles), beta decay (emission of beta particles), and gamma decay (emission of gamma rays).
- Nuclear Reactions: Processes involving changes in the composition or structure of atomic nuclei, such as nuclear fusion and fission.
The study of atoms and nuclei is crucial in various fields, including chemistry, physics, and nuclear science. It forms the foundation for understanding the behavior of matter and the principles behind energy production, radiation, and nuclear technology.
The syllabus for the “Atoms and Nuclei” topic in the NEET (National Eligibility cum Entrance Test) physics section is based on the concepts of atomic structure, atomic models, nuclear structure, and properties of atomic and nuclear particles. Here’s a comprehensive overview of the integrated course syllabus for NEET physics in relation to “Atoms and Nuclei”:
- Atomic Structure:
- Discovery of the electron, proton, and neutron.
- Atomic models: Thomson’s model, Rutherford’s model, Bohr’s model.
- Bohr’s theory of hydrogen-like atoms.
- Quantum numbers and their significance.
- Electronic configuration of atoms.
- The Pauli exclusion principle, Hund’s rule, and Aufbau principle.
- Shapes of atomic orbitals (s, p, d, f) and their orientations.
- X-rays:
- Production of X-rays and X-ray spectra.
- Bragg’s law and X-ray diffraction.
- Dual Nature of Matter and Radiation:
- Wave-particle duality.
- De Broglie wavelength and its significance.
- Davisson-Germer experiment.
- Nucleus:
- Atomic mass, atomic number, isotopes, and isobars.
- Composition and size of the nucleus.
- Nuclear forces and their properties.
- Binding energy and mass defect.
- Radioactivity and radioactive decay.
- Alpha, beta, and gamma decay processes.
- Nuclear reactions and their balancing.
- Radioactivity:
- Laws of radioactive decay (decay constant, half-life, mean-life).
- Growth and decay of a radioactive substance.
- Radioactive series and equilibrium.
- Artificial transmutation of elements.
- Fission and fusion processes.
- Nuclear Energy:
- Energy released in nuclear reactions.
- Nuclear reactors and their working principles.
- Nuclear power plants and their components.
- Nuclear weapons and their impact.
- Radiation Detectors:
- Ionization chambers, GM counters, and scintillation counters.
- Principles of operation and uses of radiation detectors.
It’s important to note that this is a general overview of the topics covered in the “Atoms and Nuclei” section of NEET physics. The syllabus may vary slightly from year to year, so it’s advisable to refer to the official NEET website or relevant study materials for the most up-to-date information.
What is Required NEET-PHYSICS-SYLLABUS Atoms and Nuclei
The NEET physics syllabus for the “Atoms and Nuclei” topic includes the following subtopics and concepts:
- Atomic Structure:
- Discovery of the electron, proton, and neutron.
- Rutherford’s model of the atom.
- Bohr’s theory of hydrogen-like atoms.
- Quantum numbers and their significance.
- Electronic configuration of atoms.
- Pauli exclusion principle, Hund’s rule, and Aufbau principle.
- Atomic Spectra:
- Line spectra and their characteristics.
- Bohr’s theory and the concept of energy levels.
- Explanation of atomic spectra using energy transitions.
- X-rays:
- Production of X-rays.
- Properties of X-rays.
- X-ray spectra and continuous X-ray emissions.
- Applications of X-rays.
- Dual Nature of Radiation:
- Wave-particle duality.
- Particle nature of electromagnetic radiation.
- Photoelectric effect and its explanation.
- Einstein’s photoelectric equation.
- Nuclei and Radioactivity:
- Atomic mass, atomic number, isotopes, and isobars.
- Composition and size of the nucleus.
- Nuclear forces and their properties.
- Radioactivity and types of radioactive decay.
- Laws of radioactive decay (decay constant, half-life, mean-life).
- Alpha, beta, and gamma decay processes.
- Radioactive decay series and equilibrium.
- Artificial transmutation of elements.
- Nuclear Energy:
- Binding energy and mass defect.
- Nuclear reactions and their balancing.
- Nuclear fission and fusion.
- Energy released in nuclear reactions.
- Nuclear reactors and nuclear power plants.
- Radiation Detectors:
- Principles and working of ionization chambers, GM counters, and scintillation counters.
- Uses of radiation detectors.
It’s important to note that the NEET physics syllabus is subject to change, and it’s recommended to refer to the official NEET website or relevant study materials for the most up-to-date and detailed information.
When is Required NEET-PHYSICS-SYLLABUS Atoms and Nuclei
The “Atoms and Nuclei” topic is part of the NEET physics syllabus. NEET is a national-level entrance examination conducted in India for admission to undergraduate medical (MBBS/BDS) and dental courses in various government and private colleges.
The specific timing or schedule for studying the “Atoms and Nuclei” topic within the NEET physics syllabus may vary based on the individual’s study plan and preparation timeline. However, it is generally advisable to cover the entire NEET physics syllabus, including the “Atoms and Nuclei” topic, well in advance of the examination.
To effectively prepare for NEET physics, it is recommended to follow a structured study plan, allocate sufficient time for each topic, and make use of reliable study materials, textbooks, and online resources. Additionally, practicing previous years’ question papers and taking mock tests can help assess your understanding and improve your exam readiness.
For the most accurate and updated information on the NEET physics syllabus and its timing, it is best to refer to the official NEET website or consult authoritative study resources specifically designed for NEET preparation.
Where is Required NEET-PHYSICS-SYLLABUS Atoms and Nuclei
The “Atoms and Nuclei” topic is part of the NEET physics syllabus, which is a national-level entrance examination conducted in India for admission to undergraduate medical (MBBS/BDS) and dental courses. The syllabus for NEET physics, including the “Atoms and Nuclei” topic, is defined by the National Testing Agency (NTA), the conducting body of the NEET exam.
You can find the detailed NEET physics syllabus, including the “Atoms and Nuclei” topic, on the official website of the National Testing Agency (NTA) or the official NEET website. The syllabus outlines the specific concepts, subtopics, and knowledge areas that candidates are expected to study and be familiar with for the physics section of the NEET exam.
In addition to the official sources, there are various NEET preparation books and study materials available in the market that provide comprehensive coverage of the NEET physics syllabus, including the “Atoms and Nuclei” topic. These resources can be helpful in understanding the concepts, practicing questions, and preparing effectively for the NEET examination.
It’s important to note that the NEET physics syllabus may undergo periodic revisions or updates. Therefore, it is advisable to refer to the official NEET website or the NTA’s official notification for the most up-to-date and accurate information regarding the NEET physics syllabus and its specific topics like “Atoms and Nuclei”.
How is Required NEET-PHYSICS-SYLLABUS Atoms and Nuclei
The “Atoms and Nuclei” topic in the NEET physics syllabus is designed to assess candidates’ understanding of the atomic structure, nuclear physics, and related concepts. To effectively prepare for this topic, it is important to have a clear understanding of the key concepts and subtopics mentioned earlier.
Here’s a general approach to studying the “Atoms and Nuclei” topic for NEET physics:
- Review Atomic Structure: Start by revising the fundamental concepts of atomic structure, including the discovery of subatomic particles (electrons, protons, and neutrons) and the arrangement of these particles in an atom. Understand the different atomic models proposed by scientists such as Thomson, Rutherford, and Bohr.
- Study Quantum Mechanics: Familiarize yourself with the basics of quantum mechanics as it relates to atomic structure. Learn about quantum numbers, electron configurations, and the principles that govern electron arrangement in orbitals.
- Explore Atomic Spectra: Understand the phenomenon of atomic spectra and how it relates to the energy levels and transitions within an atom. Study the Bohr model’s explanation of atomic spectra and the concept of discrete energy levels.
- Delve into Nuclei and Radioactivity: Dive into the study of the nucleus, including its composition, size, and nuclear forces. Learn about isotopes, isobars, and the concept of radioactivity. Understand the types of radioactive decay (alpha, beta, gamma) and the laws governing radioactive decay, such as half-life and mean-life.
- Study Nuclear Energy: Explore the concepts of binding energy, mass defect, and the energy released during nuclear reactions. Learn about nuclear fission and fusion, their processes, and their applications. Familiarize yourself with the working principles of nuclear reactors and nuclear power plants.
- Understand Radiation Detectors: Gain knowledge about the principles and working mechanisms of radiation detectors such as ionization chambers, GM counters, and scintillation counters. Learn about their applications and uses in various fields.
- Practice and Solve Problems: To reinforce your understanding of the concepts and improve problem-solving skills, practice a wide range of questions and numerical problems related to atoms, nuclei, and radioactivity. Use previous years’ question papers and NEET-specific study materials for targeted practice.
Remember to allocate sufficient time to each subtopic, revise regularly, and make use of reliable textbooks, reference materials, and online resources that cover the NEET physics syllabus comprehensively. Practice solving numerical problems and answer MCQs to enhance your speed and accuracy.
Lastly, it’s important to stay updated with any changes or revisions to the NEET physics syllabus, so regularly check the official NEET website or consult reputable sources for the most accurate and updated information.
Case Study on NEET-PHYSICS-SYLLABUS Atoms and Nuclei
Case Study: The Discovery of the Atomic Nucleus
In the early 20th century, several groundbreaking experiments led to the discovery of the atomic nucleus, revolutionizing our understanding of the structure of atoms. This case study focuses on three key experiments that played a crucial role in uncovering the existence of the atomic nucleus: the Geiger-Marsden experiment, the Rutherford gold foil experiment, and the experiments on isotopes by Frederick Soddy.
Experiment 1: Geiger-Marsden Experiment Ernest Rutherford, along with his colleagues Hans Geiger and Ernest Marsden, conducted the Geiger-Marsden experiment in 1909. They aimed to investigate the structure of atoms by bombarding a thin gold foil with alpha particles. Alpha particles are positively charged particles emitted by certain radioactive substances.
The experiment involved shooting a beam of alpha particles at the gold foil and observing their scattering pattern. According to the prevailing Thomson model of the atom, which proposed that the positive charge in an atom was spread out uniformly, the alpha particles should pass through the foil with minimal deflection.
However, the results of the experiment were unexpected. Some of the alpha particles experienced significant deflection, with a small fraction even bouncing back in the direction from which they came. This indicated that the positive charge in an atom was concentrated in a small region, which became known as the atomic nucleus.
Experiment 2: Rutherford Gold Foil Experiment Building upon the findings of the Geiger-Marsden experiment, Rutherford conducted the famous gold foil experiment in 1911. The experiment involved bombarding a thin gold foil with alpha particles and studying their scattering pattern.
Based on the observations, Rutherford proposed a new atomic model that accounted for the unexpected deflections. He theorized that atoms consisted mostly of empty space, with a tiny, dense, positively charged nucleus at the center. The alpha particles, being positively charged, would experience repulsion when they approached the positively charged nucleus, leading to deflection or scattering.
This groundbreaking experiment provided strong evidence for the existence of the atomic nucleus and established the nucleus as the central core of the atom.
Experiment 3: Experiments on Isotopes by Frederick Soddy Frederick Soddy, a contemporary of Rutherford, conducted experiments on radioactivity and isotopes. Soddy investigated various radioactive elements and observed that they decayed into different elements over time.
Through his experiments, Soddy identified that atoms of the same element could have different masses, known as isotopes. He discovered that isotopes of an element had the same number of protons but different numbers of neutrons in their nuclei.
Soddy’s work on isotopes further solidified the understanding of atomic structure and contributed to the development of the modern understanding of nuclear chemistry.
Conclusion: The case study highlights how a series of experiments on atoms and nuclei led to the discovery of the atomic nucleus and transformed our understanding of atomic structure. The Geiger-Marsden experiment, Rutherford’s gold foil experiment, and Soddy’s experiments on isotopes played pivotal roles in unraveling the nature of atoms and confirming the existence of the atomic nucleus. These findings laid the foundation for further advancements in nuclear physics and shaped our understanding of the fundamental building blocks of matter.
White paper on NEET-PHYSICS-SYLLABUS Atoms and Nuclei
Title: Exploring the Subatomic World: A White Paper on Atoms and Nuclei
Abstract: This white paper delves into the intricate realm of atoms and nuclei, providing an in-depth analysis of their structure, properties, and significance in the world of physics. Understanding the fundamental constituents of matter is essential for unraveling the mysteries of the universe and advancing scientific knowledge. By exploring the historical development, theoretical foundations, and experimental discoveries, this white paper aims to provide a comprehensive overview of atoms and nuclei and their relevance in various fields.
- Introduction:
- Importance of studying atoms and nuclei in physics.
- Overview of the historical context and milestones in atomic and nuclear research.
- Atomic Structure:
- Subatomic particles: electrons, protons, and neutrons.
- Development of atomic models: Thomson, Rutherford, and Bohr models.
- Quantum mechanics and electron configurations.
- Nuclear Structure:
- Composition and properties of the atomic nucleus.
- Nuclear forces and binding energy.
- Isotopes and isobars: Understanding nuclear stability and radioactive decay.
- Atomic Spectra and Quantum Mechanics:
- Emission and absorption spectra of atoms.
- Bohr’s theory and energy levels.
- Quantum mechanical description of atomic spectra.
- Radioactivity and Nuclear Reactions:
- Types of radioactive decay: alpha, beta, and gamma decay.
- Radioactive decay laws: decay constant, half-life, and mean-life.
- Nuclear reactions: fusion, fission, and transmutation.
- Applications of Atoms and Nuclei:
- Nuclear energy and power generation.
- Radiopharmaceuticals and medical imaging.
- Carbon dating and archaeological studies.
- Nuclear research and particle accelerators.
- Modern Developments and Future Perspectives:
- Advances in subatomic particle research (e.g., discovery of new particles).
- Current trends in nuclear physics and atomic spectroscopy.
- Exploration of exotic nuclei and fundamental interactions.
- Technological Challenges and Safety Considerations:
- Nuclear safety measures and waste management.
- Risks associated with nuclear energy and radiation exposure.
- Future prospects for safer and sustainable nuclear technologies.
- Conclusion:
- Recapitulation of the key findings and concepts discussed.
- Implications of understanding atoms and nuclei for scientific progress.
- Future research directions and the importance of continued exploration.
This white paper serves as a valuable resource for scientists, researchers, educators, and enthusiasts seeking a comprehensive understanding of atoms and nuclei. By providing a synthesis of historical context, theoretical foundations, experimental findings, and practical applications, it aims to inspire further research and advancements in the fascinating field of atomic and nuclear physics.