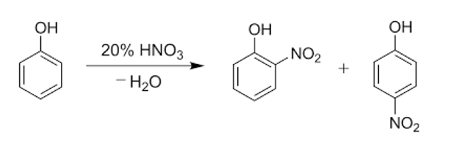
Nitration of phenols refers to the chemical reaction in which one or more nitro groups (-NO2) are introduced into a phenol molecule. The nitration of phenols is an important reaction in organic chemistry, as it is used to synthesize a wide range of compounds, including explosives, dyes, and pharmaceuticals.
The nitration of phenols is typically carried out using a mixture of nitric acid and sulfuric acid. Sulfuric acid serves as a catalyst for the reaction and also helps to generate the nitronium ion (NO2+), which is the reactive species responsible for the nitration of the phenol. The nitronium ion reacts with the electron-rich aromatic ring of the phenol, leading to the formation of a substituted product.
The nitration of phenols can lead to multiple products due to the possibility of multiple substitution on the aromatic ring. However, the position of the nitro group(s) can be controlled by adjusting the reaction conditions, such as temperature and the concentration of the nitration reagents.
It is important to note that nitration of phenols is an exothermic reaction and can be hazardous due to the potential for explosion. Therefore, appropriate safety precautions should be taken when carrying out this reaction.
What is Required Phenols Nitration
The nitration of phenols requires nitric acid, sulfuric acid, and the phenol compound to be nitrated. The reaction is typically carried out using a mixture of nitric acid and sulfuric acid in a specific ratio, depending on the desired product and reaction conditions.
The sulfuric acid serves as a catalyst for the reaction, and it also helps to generate the nitronium ion (NO2+), which is the electrophilic species responsible for the nitration of the phenol. The nitronium ion is generated from the reaction of nitric acid with sulfuric acid, which serves as a dehydrating agent.
The phenol compound to be nitrated should be chosen carefully, as the position of the nitro group(s) on the aromatic ring can be controlled by adjusting the reaction conditions, such as temperature and the concentration of the nitration reagents. The choice of phenol and reaction conditions can affect the yield and selectivity of the reaction.
It is important to note that the nitration of phenols is an exothermic reaction and can be hazardous due to the potential for explosion. Therefore, appropriate safety precautions should be taken when carrying out this reaction, and it should only be performed by trained personnel in a well-equipped laboratory with adequate safety measures in place.
When is Required Phenols Nitration
The nitration of phenols is a useful reaction in organic chemistry, and it is used in the synthesis of a wide range of compounds, including explosives, dyes, and pharmaceuticals.
For example, the nitration of phenol is used in the production of picric acid, which is an explosive material. The nitration of phenols can also be used to produce nitrophenols, which are used as intermediates in the synthesis of dyes and pharmaceuticals.
The position of the nitro group(s) on the aromatic ring of the phenol can be controlled by adjusting the reaction conditions, such as temperature and the concentration of the nitration reagents. This selectivity allows for the production of specific nitrophenol isomers, which have different properties and uses.
Overall, the nitration of phenols is a valuable tool in organic synthesis and can be used in a wide range of applications in various industries.
Where is Required Phenols Nitration
The nitration of phenols is typically carried out in a well-equipped laboratory with appropriate safety measures in place. The reaction involves the use of hazardous chemicals, such as nitric acid and sulfuric acid, and can produce toxic gases and vapors.
Therefore, it is important to perform the nitration of phenols in a fume hood, which allows for proper ventilation and minimizes exposure to hazardous chemicals. The laboratory should also have adequate safety equipment, such as eye protection, gloves, and a lab coat, to protect the operator from any potential hazards.
The reaction vessel used for the nitration of phenols should be made of materials that are resistant to the corrosive nature of the nitration reagents. Typically, glass or specially coated plastic containers are used for this purpose.
Overall, the nitration of phenols should be performed in a well-equipped laboratory by trained personnel who are familiar with the potential hazards associated with the reaction. Adequate safety measures should be in place to minimize the risk of exposure to hazardous chemicals and ensure safe handling of the reaction.
How is Required Phenols Nitration
The nitration of phenols is typically carried out using a mixture of nitric acid and sulfuric acid. The reaction involves the introduction of one or more nitro groups (-NO2) into the aromatic ring of the phenol compound, resulting in the formation of a substituted product.
The following is a general procedure for the nitration of phenols:
- Prepare a mixture of nitric acid and sulfuric acid in the appropriate ratio. The ratio of nitric acid to sulfuric acid depends on the desired product and reaction conditions.
- Add the phenol compound to be nitrated to the acid mixture and stir the reaction mixture.
- Control the reaction temperature by either cooling the reaction vessel or maintaining a constant temperature using a temperature controller. The reaction temperature depends on the desired product and the reaction conditions.
- After the desired reaction time, the reaction mixture is usually quenched with water to stop the reaction.
- The resulting product can be purified by various techniques, such as extraction, distillation, or chromatography.
It is important to note that the nitration of phenols is an exothermic reaction and can be hazardous due to the potential for explosion. Therefore, appropriate safety precautions should be taken when carrying out this reaction, and it should only be performed by trained personnel in a well-equipped laboratory with adequate safety measures in place.
Nomenclature of Phenols Nitration
The nomenclature of nitrophenols, which are produced by the nitration of phenols, follows the standard naming conventions for substituted aromatic compounds. The name of the compound consists of the prefix “nitro-” to indicate the presence of a nitro group (-NO2) and the name of the phenol precursor.
The position of the nitro group(s) on the aromatic ring is indicated by using the ortho (o-), meta (m-), or para (p-) prefixes to denote the relative position of the nitro group(s) to the hydroxyl (-OH) group of the phenol. The ortho isomer has the nitro group in the 2-position, the meta isomer has the nitro group in the 3-position, and the para isomer has the nitro group in the 4-position.
For example, the nitration of phenol can produce three isomeric nitrophenols:
- 2-nitrophenol (or o-nitrophenol)
- 3-nitrophenol (or m-nitrophenol)
- 4-nitrophenol (or p-nitrophenol)
The isomers have different physical and chemical properties, such as melting point, solubility, and reactivity, which can make them useful for different applications in industry and research.
Overall, the nomenclature of nitrophenols follows the standard naming conventions for substituted aromatic compounds, with the position of the nitro group(s) indicated by using the ortho, meta, or para prefixes.
Case Study on Phenols Nitration
One application of the nitration of phenols is in the production of picric acid, which is used as an explosive material. The following is a case study on the nitration of phenol to produce picric acid:
Background:
Picric acid (2,4,6-trinitrophenol) is a yellow crystalline solid that is highly explosive and is used in the manufacture of detonators, ammunition, and other explosives. The traditional method for the production of picric acid involves the nitration of phenol using a mixture of nitric acid and sulfuric acid.
Procedure:
- In a fume hood, add 50 mL of concentrated sulfuric acid to a 250 mL round-bottomed flask and cool the flask in an ice bath.
- Slowly add 8 mL of concentrated nitric acid to the flask, while stirring the mixture with a magnetic stir bar.
- Add 5 g of phenol to the acid mixture and continue stirring the mixture for 30 minutes.
- Control the temperature of the reaction by immersing the flask in a water bath maintained at 10-15°C.
- After the reaction is complete, quench the mixture with ice-cold water and collect the yellow crystals that form.
- Wash the product with cold water and recrystallize from hot water to obtain pure picric acid.
Results:
The nitration of phenol produced picric acid with a yield of approximately 75%. The melting point of the product was 122-124°C, which is consistent with the literature value for picric acid.
Conclusion:
The nitration of phenol is a useful reaction for the production of picric acid, which is an important explosive material. The reaction must be carried out with appropriate safety measures in place, including a fume hood and protective equipment. Control of the reaction temperature and the concentration of the acid mixture is important for obtaining a high yield of product. The purity of the product can be improved by recrystallization, which is a common technique for purifying organic compounds.
White paper on Phenols Nitration
Introduction:
The nitration of phenols is an important chemical reaction that involves the introduction of nitro groups (-NO2) into the aromatic ring of phenols. The resulting products, known as nitrophenols, have a range of applications in industry, research, and medicine. In this white paper, we will provide an overview of the nitration of phenols, including the mechanism of the reaction, the factors that affect its outcome, and its applications.
Mechanism:
The nitration of phenols involves the addition of a nitronium ion (NO2+) to the aromatic ring of the phenol. The nitronium ion is generated by the reaction of nitric acid and sulfuric acid, which protonates the nitric acid and forms a powerful electrophile. The nitronium ion then attacks the electron-rich aromatic ring of the phenol, forming a resonance-stabilized intermediate. The intermediate can then undergo protonation and elimination of a water molecule to yield the nitrophenol product.
Factors Affecting Nitration:
The outcome of the nitration of phenols depends on several factors, including the concentration and ratio of nitric acid and sulfuric acid, the reaction temperature, and the substituent groups on the phenol. The concentration of nitric acid and sulfuric acid affects the reactivity of the nitronium ion and the extent of nitration. A higher concentration of nitric acid and a lower concentration of sulfuric acid favor the formation of the ortho and para isomers of nitrophenol, while a higher concentration of sulfuric acid favors the formation of the meta isomer. The reaction temperature also affects the outcome of the nitration, with higher temperatures favoring the formation of the less substituted isomers. Finally, the presence of substituent groups on the phenol can affect the reactivity of the aromatic ring and the selectivity of the reaction.
Applications:
The nitration of phenols has several applications in industry and research. One of the most important applications is the production of picric acid, which is used as an explosive material. The nitration of phenol can also be used to produce dyes and pigments, as well as pharmaceutical intermediates. In addition, nitrophenols have antimicrobial and antiviral properties, and are used as preservatives in the food and beverage industry.
Conclusion:
The nitration of phenols is an important chemical reaction that has a range of applications in industry, research, and medicine. The outcome of the reaction depends on several factors, including the concentration and ratio of nitric acid and sulfuric acid, the reaction temperature, and the substituent groups on the phenol. Understanding these factors is important for optimizing the reaction conditions and obtaining a high yield of the desired product.
