
Nitrogen is a chemical element with the symbol N and atomic number 7. It is a colorless, odorless, and tasteless gas that makes up about 78% of the Earth’s atmosphere by volume. Nitrogen is an essential element for life, as it is a component of amino acids, which are the building blocks of proteins.
Nitrogen is also used in many industrial processes, such as in the production of fertilizers, explosives, and ammonia. It can be obtained from the air by separating it from oxygen using processes such as fractional distillation, membrane separation, or pressure swing adsorption.
Nitrogen has several isotopes, with nitrogen-14 being the most abundant in nature. Nitrogen-15 is used in various scientific applications, such as in tracer studies in biology and chemistry, as well as in nuclear magnetic resonance (NMR) spectroscopy.
What is Required Nitrogen
Required nitrogen refers to the amount of nitrogen that is needed for a particular plant or crop to grow and develop properly. Nitrogen is one of the essential macronutrients that plants require, and it is necessary for the production of chlorophyll, which is used in photosynthesis.
The required amount of nitrogen can vary depending on the type of plant or crop, the soil conditions, and the stage of growth. Plants that are in their early stages of growth, for example, require more nitrogen than mature plants.
To determine the required nitrogen for a particular plant or crop, soil testing can be done to assess the amount of nitrogen that is already present in the soil. Based on the results of the soil test, additional nitrogen may need to be added through fertilizers or other methods to ensure that the plant has access to the required amount of nitrogen for optimal growth and development.
Liquid nitrogen

“LN2” diverts here. For the rapid rail route line in France, see LGV Atlantique. For the normal logarithm of 2, see Regular logarithm of 2.
Fluid nitrogen — LN2 — is nitrogen in a fluid state at low temperature. Fluid nitrogen has a limit of about −195.8 °C (−320 °F; 77 K). It is delivered mechanically by partial refining of fluid air. It is a dismal, low thickness fluid that is broadly utilized as a coolant.
Nitrogen cycle
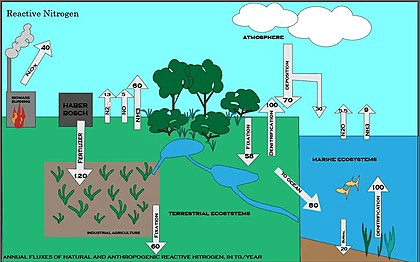
The nitrogen cycle is the biogeochemical cycle by which nitrogen is changed over into different compound structures as it circles among air, earthbound, and marine biological systems. The change of nitrogen can be brought out through both organic and actual cycles. Significant cycles in the nitrogen cycle incorporate obsession, ammonification, nitrification, and denitrification. Most of Earth’s climate (78%) is air nitrogen, making it the biggest wellspring of nitrogen. In any case, air nitrogen has restricted accessibility for natural use, prompting a shortage of usable nitrogen in many sorts of biological systems.
The nitrogen cycle is exceptionally compelling to environmentalists since nitrogen accessibility can influence the pace of key biological system processes, including essential creation and disintegration. Human exercises like non-renewable energy source burning, utilization of fake nitrogen manures, and arrival of nitrogen in wastewater have emphatically adjusted the worldwide nitrogen cycle. Human change of the worldwide nitrogen cycle can adversely influence the indigenous habitat framework and furthermore human wellbeing.
Solid nitrogen

Strong nitrogen is various strong types of the component nitrogen, first saw in 1884. Strong nitrogen is essentially the subject of scholastic exploration, yet low-temperature, low-pressure strong nitrogen is a significant part of bodies in the external Nearby planet group and high-temperature, high-pressure strong nitrogen is a strong unstable, with higher energy thickness than some other non-atomic material.
Nitrogen fixation
Nitrogen obsession or natural nitrogen obsession (BNF) is a synthetic cycle by which sub-atomic nitrogen (N2), which has areas of strength for a covalent bond, is changed over into smelling salts (NH3) or related nitrogenous mixtures, commonly in soil or sea-going frameworks yet in addition in industry. The nitrogen in air is sub-atomic dinitrogen, a somewhat nonreactive particle that is metabolically pointless to everything except a couple of microorganisms. Natural nitrogen obsession or diazotrophy is a significant microorganism interceded process that changes over dinitrogen (N2) gas to smelling salts (NH3) utilizing the nitrogenase protein complex (Nif).
Nitrogen obsession is vital for life in light of the fact that proper inorganic nitrogen compounds are expected for the biosynthesis of all nitrogen-containing natural mixtures, for example, amino acids and proteins, nucleoside triphosphates and nucleic acids. As a component of the nitrogen cycle, it is fundamental for horticulture and the production of manure. It is additionally, in a roundabout way, pertinent to the production of all nitrogen synthetic mixtures, which incorporate a few explosives, drugs, and colors.
Nitrogen obsession is done normally in soil by microorganisms named diazotrophs that incorporate microbes, like Azotobacter, and archaea. A few nitrogen-fixing microscopic organisms have harmonious associations with plant gatherings, particularly vegetables. Looser non-advantageous connections among diazotrophs and plants are frequently alluded to as affiliated, as found in nitrogen obsession with rice roots. Nitrogen obsession happens between certain termites and organisms. It happens normally in the air through NOx creation by lightning.
All natural responses including the course of nitrogen obsession are catalyzed by catalysts called nitrogenases. These catalysts contain iron, frequently with a subsequent metal, typically molybdenum however in some cases vanadium.
Case Study on Nitrogen
One case study on nitrogen involves the impact of excessive nitrogen in the environment, specifically in aquatic ecosystems. Nitrogen is an essential nutrient for plants, but excess nitrogen can cause environmental problems such as eutrophication, which is the excessive growth of algae and other aquatic plants in lakes and rivers.
In the mid-20th century, there was a rapid increase in the use of nitrogen-based fertilizers to support the growing demand for food. While these fertilizers have improved agricultural yields, excess nitrogen can leach into groundwater and surface waters, leading to the development of hypoxic or anoxic zones in aquatic ecosystems. These areas are low in dissolved oxygen, and they can negatively impact fish and other aquatic organisms.
One example of this problem is the Gulf of Mexico’s “Dead Zone,” an area of low oxygen that forms each summer. The Dead Zone is caused by nitrogen and other nutrients that are carried by the Mississippi River from agricultural lands in the Midwest to the Gulf of Mexico. These nutrients fuel the growth of algae, which eventually die and sink to the bottom, where their decomposition consumes oxygen from the water. The result is a hypoxic zone that can cause the death of fish and other organisms.
To address this problem, efforts have been made to reduce the amount of nitrogen that enters the environment. These efforts include improving agricultural practices such as precision application of fertilizers, using cover crops, and reducing tillage to minimize nitrogen runoff. Additionally, wastewater treatment plants can implement nitrogen removal technologies to reduce the amount of nitrogen that is discharged into waterways.
Overall, the case study highlights the importance of managing nitrogen use to balance the need for agricultural productivity with the need to protect the environment and human health.
White paper on Nitrogen
Title: Nitrogen: Sources, Uses, and Environmental Impact
Introduction:
Nitrogen is a ubiquitous element that plays a crucial role in the functioning of ecosystems and the global economy. It is the most abundant gas in the Earth’s atmosphere, accounting for about 78% of its composition by volume. Nitrogen is also an essential nutrient for plants, animals, and microorganisms, and it is widely used in industrial processes such as the production of fertilizers, plastics, and explosives.
Sources of Nitrogen:
The primary source of nitrogen is the Earth’s atmosphere, where it exists in the form of dinitrogen (N2) gas. Nitrogen can also be obtained from other sources, such as animal and plant waste, wastewater, and industrial emissions. In agricultural systems, nitrogen is often added to the soil through the application of fertilizers, which can be derived from natural sources such as manure or synthetic sources such as ammonia or urea.
Uses of Nitrogen:
Nitrogen has many applications in various fields, including agriculture, industry, and medicine. In agriculture, nitrogen is used to promote plant growth and increase crop yields. In the industrial sector, nitrogen is used to produce ammonia for use in fertilizers and as a feedstock for the production of various chemicals. It is also used to create a controlled atmosphere in food packaging and to cool and freeze food products. In medicine, nitrogen is used in cryotherapy to freeze and remove unwanted tissue.
Environmental Impact:
While nitrogen is essential for life, excess nitrogen in the environment can have detrimental effects. Excess nitrogen can lead to eutrophication, which is the overgrowth of algae and other aquatic plants in bodies of water. This can result in oxygen depletion in the water, which can cause fish kills and harm other aquatic organisms. Excess nitrogen can also contribute to the formation of ground-level ozone, which can have harmful effects on human health.
Conclusion:
Nitrogen is a vital element with many uses and applications, but its impact on the environment must be managed carefully to avoid negative consequences. Sustainable nitrogen management practices, such as reducing nitrogen inputs in agricultural systems, improving industrial processes to minimize nitrogen emissions, and improving wastewater treatment, can help mitigate the environmental impact of nitrogen.
