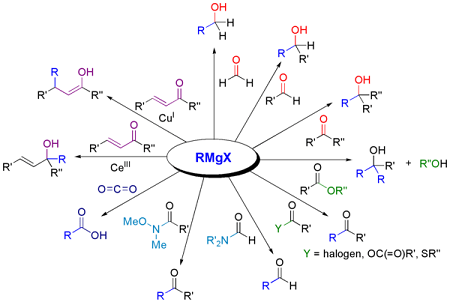
Nucleophilic addition reactions with RMgX (alkyl magnesium halides) are commonly used in organic chemistry to introduce alkyl groups into a wide variety of functional groups.
In these reactions, the RMgX reagent acts as a nucleophile, meaning that it donates a pair of electrons to an electrophilic carbon atom in a substrate. The reaction proceeds through the formation of a new carbon-carbon bond, with the alkyl group from the RMgX reagent adding to the electrophilic carbon atom.
For example, in the nucleophilic addition of RMgX to a carbonyl compound, the carbonyl carbon is electrophilic due to the polarized nature of the carbonyl group. The RMgX reagent attacks the carbonyl carbon, forming an intermediate alkoxide species, which then undergoes protonation to give the final product.
The overall reaction can be represented as follows:
R-MgX + C=O → R-C(O)-O(-)MgX(+) → R-C(O)OH + MgX(+)
where R is the alkyl group and X is a halide such as Cl or Br. This reaction is an example of a Grignard reaction, named after the French chemist François Auguste Victor Grignard, who first discovered the reagent in 1900.
What is Required Nucleophilic addition reaction with RMgX
To carry out a nucleophilic addition reaction with RMgX, there are several requirements that need to be met:
- Suitable solvent: RMgX is typically prepared in anhydrous ether, which serves as a suitable solvent for the reaction. The solvent should be dry and free of impurities to avoid unwanted side reactions.
- Dry and oxygen-free conditions: RMgX is highly reactive and sensitive to air and moisture, which can lead to unwanted side reactions. Therefore, the reaction should be carried out under dry and oxygen-free conditions using techniques such as Schlenk line or glove box.
- Electrophilic substrate: The substrate should have an electrophilic carbon atom that can react with the nucleophilic RMgX reagent. Common examples of electrophilic substrates include carbonyl compounds, epoxides, and alkyl halides.
- Appropriate temperature: The reaction should be carried out at an appropriate temperature, which is usually room temperature or slightly below. High temperatures can lead to unwanted side reactions or decomposition of the RMgX reagent.
- Proper stoichiometry: The reaction requires the appropriate stoichiometry of the RMgX reagent and the substrate. The ratio of RMgX to the substrate depends on the reactivity and functional groups present in the substrate.
By following these requirements, nucleophilic addition reactions with RMgX can be carried out effectively to introduce alkyl groups into a wide variety of functional groups.
When is Required Nucleophilic addition reaction with RMgX
Nucleophilic addition reactions with RMgX (alkyl magnesium halides) are commonly used in organic chemistry for a variety of synthetic transformations. Some examples of when nucleophilic addition reactions with RMgX are required include:
- Synthesis of alcohols: RMgX can be used to add an alkyl group to carbonyl compounds such as aldehydes and ketones, resulting in the formation of primary and secondary alcohols, respectively.
- Preparation of Grignard reagents: RMgX itself is a Grignard reagent, and can be used to prepare other Grignard reagents such as phenylmagnesium bromide.
- Synthesis of organometallic compounds: RMgX can be used to form other organometallic compounds such as lithium alkyls, by reacting with LiX.
- Synthesis of carboxylic acids: RMgX can be used to add an alkyl group to carbon dioxide, resulting in the formation of carboxylic acids.
- Formation of carbon-carbon bonds: RMgX can be used to form carbon-carbon bonds by reacting with other electrophilic compounds such as epoxides, alkyl halides, and alkynes.
Overall, nucleophilic addition reactions with RMgX are a powerful tool for introducing alkyl groups into a wide variety of functional groups, and are used extensively in organic synthesis.
Where is Required Nucleophilic addition reaction with RMgX
Nucleophilic addition reactions with RMgX (alkyl magnesium halides) can be carried out in a variety of settings, including academic research labs, industrial settings, and pharmaceutical companies.
These reactions are commonly used in organic chemistry for the synthesis of a wide range of organic compounds, including pharmaceuticals, natural products, and materials. The reactions are typically carried out in a well-equipped laboratory, using appropriate safety measures to handle the highly reactive and moisture-sensitive RMgX reagent.
In academic research labs, nucleophilic addition reactions with RMgX are often used to explore new synthetic routes to organic compounds and to study the mechanisms of organic reactions. In industry, the reactions are used for the large-scale production of organic compounds, including pharmaceutical intermediates and specialty chemicals.
Pharmaceutical companies use nucleophilic addition reactions with RMgX to synthesize drug candidates and to modify existing drug molecules to improve their pharmacological properties. The reactions are also used to produce chiral compounds, which are important in the pharmaceutical industry for their ability to selectively interact with biological targets.
Overall, nucleophilic addition reactions with RMgX are widely used in organic chemistry in various settings, including academic research labs, industrial settings, and pharmaceutical companies, to synthesize a wide range of organic compounds.
How is Required Nucleophilic addition reaction with RMgX
The general mechanism for a nucleophilic addition reaction with RMgX involves the attack of the nucleophilic carbon atom of the RMgX reagent on the electrophilic carbon atom of the substrate, followed by protonation or hydrolysis to form the final product.
The reaction typically proceeds in three steps:
- Formation of the Grignard reagent: RMgX is typically prepared by reacting an alkyl or aryl halide with magnesium metal in anhydrous ether. The Grignard reagent can then be purified and used in the nucleophilic addition reaction.
- Nucleophilic attack: The nucleophilic carbon atom of the RMgX reagent attacks the electrophilic carbon atom of the substrate, resulting in the formation of a new carbon-carbon bond. The reaction is typically carried out in a suitable solvent, such as anhydrous ether or tetrahydrofuran, under dry and oxygen-free conditions.
- Protonation or hydrolysis: After the nucleophilic addition step, the intermediate is typically protonated or hydrolyzed to form the final product. The choice of protonating or hydrolyzing agent depends on the specific reaction conditions and the functional groups present in the substrate.
The overall reaction can be represented as:
RMgX + Electrophilic Substrate → Intermediate → Final Product
Some common examples of nucleophilic addition reactions with RMgX include the synthesis of alcohols, carboxylic acids, and organometallic compounds, as well as the formation of carbon-carbon bonds. The reactions are widely used in organic synthesis to introduce alkyl groups into a wide variety of functional groups.
Nomenclature of Nucleophilic addition reaction with RMgX
The nomenclature of nucleophilic addition reactions with RMgX (alkyl magnesium halides) is typically based on the name of the Grignard reagent and the substrate used in the reaction.
The Grignard reagent is named by combining the name of the alkyl or aryl group with the name of the halogen and the word “Grignard.” For example, ethylmagnesium bromide is the Grignard reagent formed from ethyl bromide and magnesium metal.
The substrate used in the reaction is named according to the standard IUPAC rules for naming organic compounds. For example, formaldehyde is named methanal, and acetone is named propan-2-one.
When the Grignard reagent reacts with the substrate to form a new product, the name of the product is typically based on the functional group that is formed. For example, when ethylmagnesium bromide reacts with formaldehyde, the product is ethyl alcohol (also known as ethanol).
In some cases, the name of the product may include a prefix or suffix to indicate the nature of the reaction. For example, in the reaction of ethylmagnesium bromide with benzaldehyde, the product is known as a secondary alcohol, and is named (S)-1-phenylethanol.
Overall, the nomenclature of nucleophilic addition reactions with RMgX follows the standard rules for naming organic compounds, with the addition of the name of the Grignard reagent and any prefixes or suffixes to indicate the nature of the reaction or the stereochemistry of the product.
Case Study on Nucleophilic addition reaction with RMgX
One notable case study of nucleophilic addition reactions with RMgX involves the synthesis of the antimalarial drug artemisinin.
Artemisinin is a natural product isolated from the plant Artemisia annua, and is an important drug in the treatment of malaria. The natural product is produced in very low quantities in the plant, making it expensive and difficult to obtain on a large scale. In addition, the complex structure of artemisinin made it challenging to synthesize using traditional chemical methods.
In the late 1990s, a team of chemists led by K. C. Nicolaou at The Scripps Research Institute developed a new synthesis of artemisinin based on nucleophilic addition reactions with RMgX. The key step in the synthesis involved the addition of an RMgX reagent to a cyclic hemiacetal intermediate, followed by oxidation and cyclization to form the core structure of artemisinin.
The synthesis was carried out in several steps, with the key nucleophilic addition reaction involving the addition of vinyl magnesium bromide to a cyclic hemiacetal intermediate. The product of this reaction was then oxidized and cyclized to form an intermediate called dihydroartemisinin, which could be converted into artemisinin using additional chemical steps.
The synthesis was a major achievement in the field of organic chemistry, and demonstrated the power of nucleophilic addition reactions with RMgX for the synthesis of complex natural products. The synthesis has also enabled the production of artemisinin on a large scale, making it more affordable and accessible for the treatment of malaria in developing countries.
Overall, the case study of the synthesis of artemisinin highlights the importance of nucleophilic addition reactions with RMgX in organic synthesis, and their potential for the synthesis of complex natural products with important pharmacological properties.
White paper on Nucleophilic addition reaction with RMgX
Introduction:
Nucleophilic addition reactions with RMgX (alkyl magnesium halides) are an important class of organic reactions that are widely used in organic synthesis. These reactions involve the addition of a nucleophile (the RMgX reagent) to an electrophilic substrate, typically a carbonyl compound, to form a new carbon-carbon bond. In this white paper, we will explore the mechanism, applications, and recent advances in nucleophilic addition reactions with RMgX.
Mechanism:
The mechanism of nucleophilic addition reactions with RMgX involves the formation of a Grignard reagent, followed by the addition of the nucleophile to the electrophilic substrate. The Grignard reagent is formed by the reaction of an alkyl or aryl halide with magnesium metal, and has the general formula RMgX.
In the presence of an electrophilic substrate, the RMgX reagent acts as a nucleophile and attacks the electrophilic carbon atom in the substrate. This leads to the formation of an intermediate that contains a new carbon-carbon bond and a new functional group. The intermediate can then undergo additional chemical transformations, such as protonation or oxidation, to form the final product.
Applications:
Nucleophilic addition reactions with RMgX are widely used in organic synthesis for the formation of carbon-carbon bonds. These reactions are particularly useful for the synthesis of complex organic molecules, such as natural products and pharmaceuticals, that contain multiple functional groups and stereocenters.
One notable application of nucleophilic addition reactions with RMgX is the synthesis of artemisinin, an important drug in the treatment of malaria. The synthesis of artemisinin involves several nucleophilic addition reactions with RMgX, and has enabled the production of the drug on a large scale, making it more accessible for the treatment of malaria in developing countries.
Other applications of nucleophilic addition reactions with RMgX include the synthesis of terpenes, steroids, and other natural products, as well as the preparation of chiral compounds for use in pharmaceuticals and agrochemicals.
Recent advances:
Recent advances in nucleophilic addition reactions with RMgX have focused on the development of new reaction conditions and new types of substrates. For example, researchers have developed new methods for the synthesis of chiral compounds using chiral Grignard reagents, which can be used to control the stereochemistry of the final product.
In addition, researchers have explored the use of new types of substrates, such as imines and nitrones, for nucleophilic addition reactions with RMgX. These reactions have the potential to enable the synthesis of new classes of compounds with important pharmacological properties.
Conclusion:
Nucleophilic addition reactions with RMgX are an important class of organic reactions that have found widespread use in organic synthesis. These reactions are particularly useful for the synthesis of complex organic molecules and have enabled the production of important drugs, such as artemisinin, on a large scale. Recent advances in the field have focused on the development of new reaction conditions and new types of substrates, which have the potential to enable the synthesis of new classes of compounds with important pharmacological properties.
