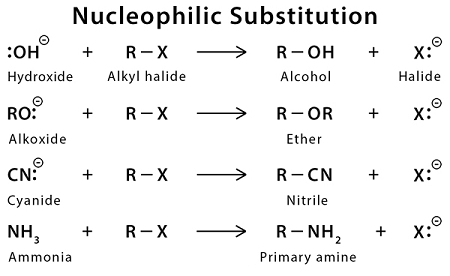
Nucleophilic substitution reactions are a type of organic reaction in which a nucleophile (a species with a lone pair of electrons) attacks an electrophilic center and replaces a leaving group. These reactions are typically observed in organic chemistry and are fundamental to many important organic reactions.
The general mechanism for a nucleophilic substitution reaction involves the attack of a nucleophile on an electrophilic carbon atom that is attached to a leaving group. The leaving group is then displaced and the nucleophile takes its place, resulting in the formation of a new compound.
There are two main types of nucleophilic substitution reactions: the SN1 and SN2 reactions. SN1 reactions are characterized by a two-step mechanism in which the leaving group is first displaced to form a carbocation intermediate, which is then attacked by a nucleophile. SN2 reactions, on the other hand, are characterized by a one-step mechanism in which the nucleophile attacks the electrophilic center and displaces the leaving group at the same time.
Nucleophilic substitution reactions are widely used in organic synthesis and are important in the production of pharmaceuticals, agrochemicals, and many other chemicals. They are also important in biochemistry, where they play a key role in the metabolism of drugs and other foreign compounds in the body.
What is Required Nucleophilic substitution reactions
There are several requirements for a successful nucleophilic substitution reaction to occur:
- A suitable nucleophile: The nucleophile must have a lone pair of electrons and be able to attack the electrophilic center of the substrate. Common nucleophiles include negatively charged ions such as hydroxide (OH-), cyanide (CN-), and halides (Cl-, Br-, I-), as well as neutral molecules such as water (H2O), ammonia (NH3), and alcohols (ROH).
- A suitable leaving group: The leaving group must be able to depart from the substrate, leaving behind a positively charged electrophilic center that can be attacked by the nucleophile. Common leaving groups include halides (Cl-, Br-, I-), sulfonate esters (e.g., tosylate, mesylate), and alkyl groups.
- A polar solvent: A polar solvent is required to stabilize the charged intermediates that are formed during the reaction. Common polar solvents include water, alcohols, and polar aprotic solvents such as dimethyl sulfoxide (DMSO) and acetonitrile.
- Appropriate reaction conditions: The reaction conditions must be chosen carefully to promote the desired mechanism (SN1 or SN2) and to avoid side reactions. Factors such as temperature, concentration, and the identity of the substrate can all affect the reaction outcome.
Overall, successful nucleophilic substitution reactions require a balance of reactivity between the nucleophile and substrate, along with appropriate reaction conditions to control the reaction outcome.
When is Required Nucleophilic substitution reactions
Nucleophilic substitution reactions are used in a variety of applications, including organic synthesis and biochemistry. Some specific examples of when nucleophilic substitution reactions are required include:
- Synthesis of pharmaceuticals: Nucleophilic substitution reactions are commonly used in the synthesis of pharmaceuticals. For example, the synthesis of aspirin involves a nucleophilic substitution reaction between salicylic acid and acetic anhydride.
- Synthesis of agrochemicals: Nucleophilic substitution reactions are also important in the synthesis of agrochemicals such as herbicides, insecticides, and fungicides.
- Biochemical reactions: Nucleophilic substitution reactions are involved in many biochemical reactions, including the metabolism of drugs and other foreign compounds in the body.
- Polymerization reactions: Nucleophilic substitution reactions can also be used in polymerization reactions to create polymers with specific properties.
- Functionalization of organic molecules: Nucleophilic substitution reactions are often used to functionalize organic molecules by adding or replacing functional groups.
Overall, nucleophilic substitution reactions are an important class of reactions in organic chemistry and have a wide range of applications in industry, research, and medicine.
Where is Required Nucleophilic substitution reactions
Nucleophilic substitution reactions are required in a variety of settings, including:
- Chemical synthesis laboratories: Nucleophilic substitution reactions are commonly used in chemical synthesis laboratories to create new compounds with specific properties. For example, pharmaceutical companies use nucleophilic substitution reactions to synthesize new drugs.
- Industrial chemical production: Nucleophilic substitution reactions are used in industrial chemical production to produce a wide range of chemicals and materials. For example, the production of plastics and other polymers often involves nucleophilic substitution reactions.
- Biochemistry labs: Nucleophilic substitution reactions are important in biochemistry labs, where they are used to study the metabolism of drugs and other foreign compounds in the body.
- Agricultural industry: Nucleophilic substitution reactions are used in the production of agrochemicals such as herbicides, insecticides, and fungicides.
- Environmental remediation: Nucleophilic substitution reactions are sometimes used in environmental remediation efforts to break down harmful chemicals and pollutants.
Overall, nucleophilic substitution reactions are an important class of reactions with a wide range of applications in various industries, research settings, and environmental contexts.
How is Required Nucleophilic substitution reactions
The mechanism of a nucleophilic substitution reaction depends on several factors, including the type of substrate, the nature of the nucleophile, and the reaction conditions. However, there are two main mechanisms of nucleophilic substitution reactions: the SN1 and SN2 reactions.
- SN1 mechanism: In an SN1 reaction, the nucleophile attacks an intermediate carbocation that is formed when the leaving group departs. The reaction proceeds through a two-step mechanism in which the leaving group departs first, leaving behind a positively charged intermediate. The nucleophile then attacks the carbocation intermediate to form the final product. This reaction is typically favored when the substrate is tertiary or allylic/benzilic, and when a polar solvent is present.
- SN2 mechanism: In an SN2 reaction, the nucleophile attacks the electrophilic center at the same time that the leaving group departs. The reaction proceeds through a one-step mechanism in which the nucleophile approaches the substrate from the opposite side of the leaving group, resulting in inversion of configuration. This reaction is typically favored when the substrate is primary or secondary, and when a non-polar aprotic solvent is present.
In general, the mechanism of a nucleophilic substitution reaction depends on the electronic and steric factors of the substrate and nucleophile, as well as the reaction conditions. The choice of mechanism can greatly affect the outcome of the reaction, including the stereochemistry of the product and the efficiency of the reaction.
Production of Nucleophilic substitution reactions
Nucleophilic substitution reactions can be produced through a variety of methods, depending on the specific reaction and application. Here are a few general methods:
- Reaction of a nucleophile with a suitable substrate: This is the most common method of producing nucleophilic substitution reactions. A nucleophile is allowed to react with a substrate that has a suitable leaving group. The reaction conditions and choice of solvent are often critical in controlling the outcome of the reaction.
- Use of phase-transfer catalysts: Phase-transfer catalysts are used to facilitate the reaction between a nucleophile and substrate that are present in different phases (e.g. organic and aqueous). These catalysts help to transport the nucleophile across the interface between the two phases, allowing the reaction to occur more efficiently.
- Use of microwave or ultrasonic irradiation: The use of microwave or ultrasonic irradiation can increase the rate of nucleophilic substitution reactions by providing energy to the reaction system. These methods are particularly useful for reactions that are slow or difficult to carry out under conventional heating.
- Enzymatic catalysis: Enzymes can be used as catalysts to facilitate nucleophilic substitution reactions in biological systems. Enzymes can increase the rate and selectivity of the reaction, making it more efficient and environmentally friendly.
Overall, the production of nucleophilic substitution reactions can be achieved through a variety of methods depending on the specific reaction and application. Factors such as reaction conditions, choice of solvent, and use of catalysts can greatly affect the efficiency and selectivity of the reaction.
Case Study on Nucleophilic substitution reactions
One example of a nucleophilic substitution reaction is the reaction between an alkyl halide and a nucleophile to form an alkyl nucleophile. Let’s take the specific case of the reaction between tert-butyl chloride and sodium methoxide.
The reaction can be represented by the following equation:
tert-butyl chloride + sodium methoxide → tert-butyl methoxide + sodium chloride
This reaction is an example of an SN2 mechanism, where the nucleophile (sodium methoxide) attacks the electrophilic carbon atom of the tert-butyl chloride, causing the leaving group (chloride ion) to depart at the same time. The reaction proceeds through a one-step mechanism and results in inversion of configuration at the carbon atom.
The reaction can be carried out in an organic solvent such as tetrahydrofuran (THF), with a catalytic amount of sodium hydroxide (NaOH) added to increase the rate of the reaction. The reaction mixture is stirred at room temperature for several hours, and the product is isolated by evaporating the solvent and purifying the residue by column chromatography.
The resulting tert-butyl methoxide can be used as a nucleophile in a variety of other reactions, such as substitution or addition reactions. This type of reaction is commonly used in organic synthesis to form new carbon-carbon or carbon-heteroatom bonds.
Overall, the nucleophilic substitution reaction between tert-butyl chloride and sodium methoxide is a useful example of the versatility and utility of nucleophilic substitution reactions in organic synthesis.
White paper on Nucleophilic substitution reactions
Introduction
Nucleophilic substitution reactions are a fundamental class of organic reactions that involve the displacement of a leaving group from an electrophilic carbon atom by a nucleophile. These reactions have a wide range of applications in organic synthesis, pharmaceuticals, and materials science. In this white paper, we will discuss the mechanism, factors influencing the reaction, and examples of nucleophilic substitution reactions.
Mechanism of Nucleophilic Substitution Reactions
There are two main mechanisms of nucleophilic substitution reactions: SN1 and SN2. In an SN1 reaction, the leaving group departs first, leaving behind a positively charged intermediate. The nucleophile then attacks the carbocation intermediate to form the final product. This reaction is typically favored when the substrate is tertiary or allylic/benzilic, and when a polar solvent is present. In an SN2 reaction, the nucleophile attacks the electrophilic center at the same time that the leaving group departs. The reaction proceeds through a one-step mechanism in which the nucleophile approaches the substrate from the opposite side of the leaving group, resulting in inversion of configuration. This reaction is typically favored when the substrate is primary or secondary, and when a non-polar aprotic solvent is present.
Factors Influencing Nucleophilic Substitution Reactions
Several factors can influence the rate and outcome of a nucleophilic substitution reaction, including the nature of the substrate, the nucleophile, the leaving group, and the reaction conditions.
- Substrate: The electronic and steric properties of the substrate play a critical role in determining the mechanism and rate of the reaction. Tertiary substrates typically undergo SN1 reactions, while primary and secondary substrates typically undergo SN2 reactions.
- Nucleophile: The strength and nucleophilicity of the nucleophile can greatly affect the rate and outcome of the reaction. Strong nucleophiles, such as hydroxide (OH-) and methoxide (CH3O-), tend to favor SN2 reactions, while weaker nucleophiles, such as water (H2O) and alcohol (ROH), tend to favor SN1 reactions.
- Leaving Group: The nature of the leaving group can also influence the rate and outcome of the reaction. Good leaving groups, such as halides (Cl-, Br-, I-), sulfonates (SO3R), and tosylates (TsO-), tend to favor SN1 reactions, while poorer leaving groups, such as hydroxyl (OH-) and alkoxide (RO-), tend to favor SN2 reactions.
- Reaction Conditions: The choice of solvent, temperature, and presence of catalysts can also greatly affect the rate and outcome of the reaction. Polar solvents tend to favor SN1 reactions, while non-polar aprotic solvents tend to favor SN2 reactions.
Examples of Nucleophilic Substitution Reactions
Nucleophilic substitution reactions have a wide range of applications in organic synthesis, pharmaceuticals, and materials science. Here are a few examples:
- Synthesis of Esters: The reaction between a carboxylic acid and an alcohol in the presence of a strong acid catalyst can produce an ester through a nucleophilic substitution reaction.
- Preparation of Amides: The reaction between a carboxylic acid and an amine can produce an amide through a nucleophilic substitution reaction.
- Drug Synthesis: The synthesis of the anti-inflammatory drug ibuprofen involves a nucleophilic substitution reaction between a halide substrate and an alkoxide nucleophile.
Conclusion
Nucleophilic substitution reactions are a fundamental class of organic reactions with a wide range of applications in organic synthesis, pharmaceuticals, and materials science. The mechanism and outcome of these reactions are influenced by several factors, including the nature of the substrate, the nucleophile, the leaving group, and the reaction conditions. Understanding these factors is critical in optimizing the reaction conditions to achieve the desired product. Examples of nucleophilic substitution reactions include the synthesis of esters and amides, as well as the production of various drugs. As a versatile and important class of reactions, nucleophilic substitution reactions continue to be an active area of research in organic chemistry.
