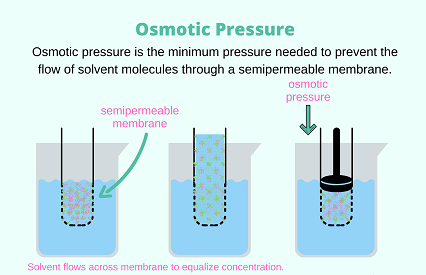
Osmotic pressure is the pressure required to prevent the flow of solvent molecules across a semipermeable membrane, due to the presence of solute molecules. In other words, it is the pressure needed to stop the flow of solvent from a region of low solute concentration to a region of high solute concentration, when the two regions are separated by a semipermeable membrane that allows the passage of solvent molecules but not solute molecules.
The osmotic pressure depends on the concentration of solute particles, the temperature, and the properties of the solvent and membrane. It can be calculated using the following equation:
Π = iMRT
where Π is the osmotic pressure, i is the van’t Hoff factor (a measure of the number of particles per molecule of solute), M is the molarity of the solution, R is the gas constant, and T is the temperature in Kelvin.
Osmotic pressure has important biological and industrial applications, such as in the regulation of water balance in cells and tissues, the purification of water and other liquids, and the production of pharmaceuticals and food products.
What is Required Osmotic pressure
Required osmotic pressure is the minimum osmotic pressure required to prevent the flow of solvent molecules from a solution to a pure solvent or another solution with a higher solvent concentration, through a semipermeable membrane.
For example, if a cell is placed in a solution that has a higher solute concentration than the cell’s cytoplasm, water will flow out of the cell through the semipermeable membrane to equalize the concentration of solute on both sides. This will cause the cell to shrink or even die if the osmotic pressure of the external solution is too high.
The required osmotic pressure can be used to calculate the minimum concentration of solutes required to maintain the integrity and function of cells and tissues, such as in intravenous solutions used in medical treatments. It can also be used in the design and operation of reverse osmosis systems for water purification, where a required osmotic pressure is applied to the feed solution to remove impurities and produce clean water.
When is Required Osmotic pressure
Required osmotic pressure is relevant in any situation where there is a semipermeable membrane separating a solution from a solvent or another solution, and there is a concentration gradient of solute molecules across the membrane.
For example, it is important in biology when considering how cells and tissues maintain their structure and function. If the osmotic pressure is too high in a solution surrounding a cell, water will flow out of the cell through the semipermeable membrane, leading to cell shrinkage or even death. Similarly, in industrial applications such as water purification, required osmotic pressure is used to design and operate reverse osmosis systems that remove impurities from a solution by applying a required pressure to the feed solution.
Required osmotic pressure is also important in chemical and physical systems where solute molecules are separated by a semipermeable membrane, and the direction of solvent flow needs to be controlled. The concept is particularly relevant in the design and operation of membrane processes, where selective transport of molecules across a membrane is used for various applications such as gas separation, water purification, and pharmaceutical manufacturing.
Where is Required Osmotic pressure
Required osmotic pressure is a fundamental concept in physical chemistry and biology and can be observed in many natural and artificial systems where there is a semipermeable membrane separating solutions of different concentrations.
In biology, required osmotic pressure is important in understanding how cells and tissues maintain their structure and function in different environments. For example, in plants, osmotic pressure is involved in the movement of water from the soil to the roots and then to the rest of the plant. In animals, osmotic pressure regulates the movement of fluids across membranes in the kidney and other organs.
In industrial applications, required osmotic pressure is used in processes such as reverse osmosis, where a semipermeable membrane is used to separate impurities from water, and in the production of pharmaceuticals and other products where selective transport across a membrane is used to purify and separate different components.
Overall, required osmotic pressure is a ubiquitous concept in many natural and artificial systems and is relevant in various fields including physical chemistry, biology, chemical engineering, and materials science.
How is Required Osmotic pressure
Required osmotic pressure can be calculated using the following equation:
Π = iMRT
where Π is the osmotic pressure, i is the van’t Hoff factor (a measure of the number of particles per molecule of solute), M is the molarity of the solution, R is the gas constant, and T is the temperature in Kelvin.
The van’t Hoff factor takes into account the number of particles that a solute molecule will dissociate into when it is dissolved in solution. For example, NaCl (table salt) dissociates into two ions in solution (Na+ and Cl-), so its van’t Hoff factor is 2.
The molarity (M) is a measure of the concentration of solute particles in solution and is defined as the number of moles of solute per liter of solution.
The gas constant (R) is a constant that relates the energy of a gas to its temperature and pressure, and has a value of 8.314 J/(mol·K).
The temperature (T) is measured in Kelvin and represents the absolute temperature of the system.
Overall, the equation relates the osmotic pressure of a solution to its concentration, temperature, and the number of particles that the solute will dissociate into in solution. By calculating the required osmotic pressure, one can determine the minimum concentration of solute required to prevent the flow of solvent across a semipermeable membrane.
Nomenclature of Osmotic pressure
The nomenclature of osmotic pressure involves several key terms that are used to describe the different components of the system. These include:
- Osmotic pressure: This is the pressure that is required to prevent the flow of solvent molecules across a semipermeable membrane from a region of low solute concentration to a region of high solute concentration.
- Semipermeable membrane: This is a membrane that allows the passage of solvent molecules, but not solute molecules. The membrane separates the solution from a pure solvent or another solution with a different solute concentration.
- Solute: This is the substance that is dissolved in the solvent to create a solution. The solute may be a solid, liquid or gas, and can be made up of one or more types of molecules.
- Solvent: This is the substance that dissolves the solute to create a solution. In many cases, the solvent is water, but it can also be other liquids or gases.
- Concentration gradient: This is the difference in concentration of solute molecules on either side of the semipermeable membrane. The solute concentration is typically higher on one side of the membrane than the other, creating a concentration gradient that drives the flow of solvent molecules.
Overall, the nomenclature of osmotic pressure reflects the key components of the system and how they interact to create the flow of solvent across a semipermeable membrane.
Case Study on Osmotic pressure
One example of the importance of osmotic pressure is in the context of human physiology. The human body is a complex system that relies on the movement of fluids and solutes across semipermeable membranes to maintain its structure and function. One important example of this is the process of osmoregulation, which involves the regulation of fluid and solute balance in the body.
One key player in osmoregulation is the kidney, which is responsible for filtering blood and producing urine. The kidneys use a process known as filtration, reabsorption, and secretion to regulate the concentration of solutes and water in the body.
During filtration, blood is passed through a semipermeable membrane in the kidneys, known as the glomerular membrane. This membrane allows water and small solutes, such as glucose and salts, to pass through, but prevents larger molecules such as proteins and blood cells from entering the filtrate.
As the filtrate moves through the kidney tubules, solutes and water are reabsorbed back into the bloodstream, while waste products and excess water are excreted in the urine. This process is tightly regulated by hormones such as antidiuretic hormone (ADH), which controls the permeability of the kidney tubules to water, and aldosterone, which regulates the reabsorption of sodium and potassium ions.
Osmotic pressure plays a key role in this process by regulating the movement of water across the semipermeable membranes in the kidney. For example, if the concentration of solutes in the blood is too high, this will create a concentration gradient that drives the movement of water out of the kidney tubules and into the bloodstream, preventing dehydration. Conversely, if the concentration of solutes in the blood is too low, this will create a concentration gradient that drives the movement of water into the kidney tubules and out of the body in the urine, preventing fluid overload.
Overall, osmotic pressure is a critical concept in the context of human physiology, and plays a key role in the regulation of fluid and solute balance in the body.
White paper on Osmotic pressure
Introduction:
Osmotic pressure is a fundamental concept in physical chemistry and plays a key role in many biological processes, including the regulation of fluid and solute balance in the body. Osmotic pressure is defined as the pressure required to prevent the flow of solvent molecules across a semipermeable membrane from a region of low solute concentration to a region of high solute concentration. In this white paper, we will provide an overview of osmotic pressure, including its definition, measurement, and applications.
Definition of Osmotic Pressure:
Osmotic pressure is defined as the pressure that must be applied to a solution to prevent the flow of solvent molecules across a semipermeable membrane from a region of low solute concentration to a region of high solute concentration. The osmotic pressure is proportional to the concentration of solute particles in the solution, as well as to the temperature and the volume of the solution.
Measurement of Osmotic Pressure:
The osmotic pressure of a solution can be measured using a variety of methods, including the use of a semipermeable membrane and a manometer. In this method, the solution is placed in a container separated from pure solvent by a semipermeable membrane. The pressure required to prevent the flow of solvent molecules across the membrane is then measured using a manometer.
Another method for measuring osmotic pressure is the freezing point depression method. In this method, the freezing point of a solution is measured and compared to that of pure solvent. The difference in freezing point is proportional to the concentration of solute particles in the solution, and can be used to calculate the osmotic pressure.
Applications of Osmotic Pressure:
Osmotic pressure has a wide range of applications in biology, chemistry, and engineering. Some of the key applications of osmotic pressure include:
- Biological osmoregulation: As discussed earlier, osmotic pressure plays a key role in the regulation of fluid and solute balance in the body. The kidneys, for example, use osmotic pressure to regulate the concentration of solutes and water in the blood.
- Food preservation: Osmotic pressure is used in the preservation of foods such as fruits and vegetables. In this process, the food is immersed in a solution of high solute concentration, which causes water to flow out of the food and into the solution, thereby reducing the water content and inhibiting the growth of microorganisms.
- Water desalination: Osmotic pressure can be used to desalinate seawater by using a semipermeable membrane to separate the salt from the water. By applying pressure to the saltwater, water molecules are forced through the membrane, leaving behind the salt.
- Chemical purification: Osmotic pressure can be used in chemical purification processes to separate solutes from solvents. In this process, a solution containing a solute is placed in contact with a solvent, and the osmotic pressure is used to separate the solute from the solvent.
Conclusion:
Osmotic pressure is a fundamental concept in physical chemistry that plays a key role in many biological, chemical, and engineering applications. By understanding the principles of osmotic pressure, scientists and engineers can develop new technologies and processes to address a wide range of challenges, from water desalination to food preservation to chemical purification. Osmotic pressure is a critical tool in the development of sustainable and efficient processes for a wide range of industries, and will continue to be an important area of research for years to come.
