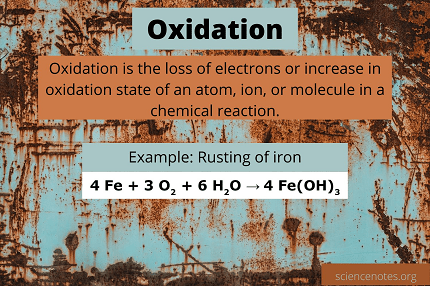
Oxidation is a chemical process in which a substance loses electrons. It is often defined as the process in which a substance reacts with oxygen to form an oxide. Oxidation reactions can also involve other elements, such as hydrogen or chlorine, and can occur without the presence of oxygen.
In an oxidation reaction, the substance that loses electrons is said to be oxidized, while the substance that gains electrons is said to be reduced. This is because the process of oxidation always involves a reduction reaction as well. The electrons that are lost during oxidation are gained by another substance, which is undergoing reduction.
Oxidation is a fundamental process in many chemical reactions, including combustion, rusting, and the metabolism of food in living organisms. It is also used in many industrial processes, such as the production of chemicals and the refining of metals.
What is Required Alkanes Oxidation
The oxidation of alkanes is a chemical process that involves the addition of oxygen or removal of hydrogen from an alkane molecule. In the case of required alkanes oxidation, it likely refers to the selective oxidation of specific alkane molecules, which may be required for a particular chemical reaction or industrial process.
One example of required alkanes oxidation is the production of industrial chemicals such as ethylene oxide and propylene oxide. These chemicals are produced through the oxidation of ethylene and propylene, respectively, using specialized catalysts and oxygen. The selectivity of the oxidation reaction is important in order to produce the desired product without the formation of unwanted byproducts.
In other cases, required alkanes oxidation may be used in the synthesis of organic compounds, such as alcohols, aldehydes, or carboxylic acids. These reactions can be achieved through the controlled oxidation of specific alkane molecules using reagents such as potassium permanganate or chromic acid.
Overall, required alkanes oxidation is an important process in many chemical reactions and industrial processes, allowing for the selective transformation of alkanes into more useful compounds.
When is Required Alkanes Oxidation
Required alkanes oxidation is a process that is used in a variety of situations where it is necessary to selectively oxidize specific alkane molecules. Some examples of when required alkanes oxidation might be used include:
- Production of industrial chemicals: As I mentioned in my previous answer, required alkanes oxidation is often used in the production of industrial chemicals such as ethylene oxide and propylene oxide.
- Synthesis of organic compounds: Required alkanes oxidation can be used in the synthesis of a wide range of organic compounds, including alcohols, aldehydes, and carboxylic acids.
- Environmental remediation: Required alkanes oxidation can be used to break down hydrocarbons that have been released into the environment as a result of oil spills or other forms of pollution.
- Petroleum refining: Required alkanes oxidation is an important process in the refining of crude oil, as it allows for the conversion of heavy hydrocarbons into lighter, more valuable products such as gasoline and diesel fuel.
Overall, required alkanes oxidation is a versatile process that can be used in a wide range of applications where it is necessary to selectively oxidize specific alkane molecules.
Where is Required Alkanes Oxidation
Required alkanes oxidation can be used in various locations, depending on the specific application. Some examples of where required alkanes oxidation might be used include:
- Chemical manufacturing facilities: Required alkanes oxidation is commonly used in the production of industrial chemicals, which are often manufactured in specialized chemical plants.
- Petroleum refineries: Required alkanes oxidation is an important process in the refining of crude oil, which takes place at large petroleum refineries.
- Environmental remediation sites: Required alkanes oxidation can be used to clean up pollution caused by hydrocarbons, such as oil spills. This can take place on land, in rivers or lakes, or in the ocean.
- Laboratories: Required alkanes oxidation may also be used in laboratory settings for the synthesis of organic compounds, as well as for research purposes.
Overall, required alkanes oxidation can be used in a wide range of locations depending on the specific application and the needs of the process or industry.
How is Required Alkanes Oxidation
The specific method used for required alkanes oxidation can vary depending on the application and the desired end product. However, in general, the process of required alkanes oxidation involves the addition of an oxidizing agent to the alkane molecule, which results in the removal of hydrogen atoms and the addition of oxygen atoms.
One common oxidizing agent used in required alkanes oxidation is potassium permanganate, which reacts with alkanes in the presence of a strong acid, such as sulfuric acid. Another common oxidizing agent is chromic acid, which can be used to selectively oxidize alkanes to alcohols, aldehydes, or carboxylic acids.
The exact conditions used for required alkanes oxidation will depend on the specific application and the desired end product. Factors such as temperature, pressure, catalysts, and reaction time may all be adjusted to optimize the oxidation reaction.
Overall, the process of required alkanes oxidation can be complex and require specialized equipment, but it is an important process for a variety of applications in industry, research, and environmental remediation.
Production of Alkanes Oxidation
Alkanes themselves are typically not produced through oxidation. Instead, alkanes are often synthesized through a process called hydrogenation, in which hydrogen gas is added to a carbon-carbon double bond, resulting in the formation of a saturated alkane molecule.
However, alkanes can be oxidized to form a variety of products, including alcohols, aldehydes, carboxylic acids, and ketones. The specific product that is formed will depend on the conditions of the oxidation reaction and the nature of the oxidizing agent used.
One important example of alkanes oxidation is the production of industrial chemicals such as ethylene oxide and propylene oxide. These chemicals are produced through the oxidation of ethylene and propylene, respectively, using specialized catalysts and oxygen.
In addition, alkanes oxidation can be used in the synthesis of a wide range of organic compounds, including pharmaceuticals, plastics, and solvents. The controlled oxidation of specific alkane molecules using reagents such as potassium permanganate or chromic acid can be used to produce a variety of functionalized products with specific properties and applications.
Overall, while alkanes themselves are not typically produced through oxidation, the oxidation of alkanes is an important process in the synthesis of a variety of useful organic compounds.
Case Study on Alkanes Oxidation
One notable case study of alkanes oxidation involves the production of ethylene oxide, a key industrial chemical used in the manufacture of a wide range of consumer products.
Ethylene oxide is produced through the catalytic oxidation of ethylene, a simple alkene molecule. The process typically involves passing a mixture of ethylene and oxygen over a silver-based catalyst, which promotes the oxidation of the ethylene to ethylene oxide.
However, this process can be challenging due to the high reactivity of ethylene, which can result in unwanted side reactions and the formation of undesired byproducts. To address this challenge, researchers have developed a range of modified catalysts and process conditions to improve the selectivity and efficiency of the oxidation process.
For example, one recent study published in the journal ACS Catalysis described the use of a novel bimetallic catalyst system to improve the selectivity of ethylene oxide production. The researchers synthesized a catalyst consisting of silver nanoparticles supported on a tin oxide substrate, and found that this catalyst exhibited higher selectivity for ethylene oxide than traditional silver-based catalysts.
In addition, researchers have also investigated the use of alternative oxidizing agents, such as hydrogen peroxide or air, to reduce the environmental impact of the ethylene oxide production process. These alternative oxidizing agents may offer advantages in terms of lower energy consumption, reduced waste, and improved selectivity.
Overall, the production of ethylene oxide through alkanes oxidation is an important case study in the development of catalytic processes for the synthesis of industrial chemicals. The ongoing research in this field highlights the importance of developing sustainable and efficient processes to meet the growing demand for these valuable chemicals.
White paper on Alkanes Oxidation
Introduction:
Alkanes are a class of hydrocarbons that are widely used in a variety of industrial and commercial applications. Alkanes are generally unreactive due to their strong carbon-carbon and carbon-hydrogen bonds, which makes them difficult to convert into other useful compounds. However, through a process known as alkanes oxidation, it is possible to introduce oxygen atoms into the alkane molecule, resulting in the formation of a range of functionalized products with diverse properties and applications.
This white paper will provide an overview of alkanes oxidation, including the principles of the process, the types of products that can be synthesized through oxidation, and some of the key applications of alkanes oxidation in industry and research.
Principles of Alkanes Oxidation:
Alkanes oxidation involves the addition of oxygen atoms to an alkane molecule, resulting in the formation of an oxygenated functional group. The specific method used for alkanes oxidation will depend on the desired end product and the conditions of the reaction. One common method for alkanes oxidation involves the use of oxidizing agents such as potassium permanganate or chromic acid, which react with alkanes in the presence of a strong acid to remove hydrogen atoms and add oxygen atoms.
The conditions of the reaction can be adjusted to control the selectivity and efficiency of the oxidation process. Factors such as temperature, pressure, catalysts, and reaction time may all be optimized to achieve the desired outcome.
Products of Alkanes Oxidation:
Alkanes oxidation can be used to synthesize a wide range of functionalized products with diverse properties and applications. Some of the key products that can be synthesized through alkanes oxidation include:
- Alcohols: Alkanes can be oxidized to form alcohols, which are important intermediates in the synthesis of a range of organic compounds. Methanol and ethanol are two common alcohols that are produced through alkanes oxidation.
- Aldehydes and Ketones: Alkanes can also be oxidized to form aldehydes and ketones, which are important intermediates in the production of pharmaceuticals, fragrances, and other fine chemicals.
- Carboxylic Acids: The controlled oxidation of alkanes can result in the formation of carboxylic acids, which are widely used in the manufacture of soaps, detergents, and other consumer products.
Applications of Alkanes Oxidation:
Alkanes oxidation has numerous applications in industry and research. Some of the key applications of alkanes oxidation include:
- Industrial Chemicals: Alkanes oxidation is used to produce a range of industrial chemicals, including ethylene oxide, propylene oxide, and glycol ethers.
- Pharmaceuticals: Alkanes oxidation is used in the synthesis of a wide range of pharmaceuticals, including antibiotics, analgesics, and anti-inflammatory drugs.
- Polymers: Alkanes oxidation is used to produce a variety of polymers, including polyesters, polycarbonates, and polyurethanes.
- Environmental Remediation: Alkanes oxidation can be used to clean up pollution caused by hydrocarbons, such as oil spills.
Conclusion:
Alkanes oxidation is a powerful process for the synthesis of functionalized products with diverse properties and applications. The principles of alkanes oxidation can be applied in a wide range of industrial and research settings, offering opportunities for the development of new and innovative products and processes. As demand for sustainable and efficient chemical synthesis continues to grow, alkanes oxidation is likely to play an increasingly important role in meeting the needs of the chemical industry.
