Oxidation of biomolecules refers to a process in which electrons are lost from a molecule, leading to a decrease in its energy content. Biomolecules such as carbohydrates, lipids, and proteins can undergo oxidation in the presence of oxygen, which is a strong oxidizing agent.
Carbohydrates, for example, can undergo oxidation to produce energy in the form of ATP through a series of reactions known as cellular respiration. In this process, glucose is oxidized in the presence of oxygen to produce carbon dioxide and water, releasing energy in the process.
Lipids can also undergo oxidation, leading to the production of energy in the form of ATP. The oxidation of lipids occurs in the mitochondria, where fatty acids are broken down through a series of reactions known as beta-oxidation.
Proteins can also undergo oxidation, which can lead to the formation of reactive oxygen species (ROS) that can cause damage to cells. However, cells have mechanisms in place to protect against the harmful effects of ROS, such as antioxidants.
Overall, oxidation of biomolecules plays a critical role in cellular metabolism, energy production, and cellular signaling. However, excessive oxidation can lead to cell damage and contribute to the development of diseases such as cancer, cardiovascular disease, and neurodegenerative diseases.
What is Required Biomolecules Oxidation
Biomolecules oxidation requires the presence of an oxidizing agent, such as oxygen, to accept electrons from the molecule being oxidized. The process of biomolecule oxidation involves the loss of electrons and a corresponding increase in the oxidation state of the molecule. This process typically involves a series of redox reactions that are coupled with the production of energy in the form of ATP.
The biomolecules that are commonly oxidized in living organisms include carbohydrates, lipids, and proteins. Carbohydrates, such as glucose, are oxidized through a process called glycolysis, which occurs in the cytoplasm of the cell. Lipids, such as fatty acids, are oxidized through a process called beta-oxidation, which occurs in the mitochondria. Proteins can also be oxidized, which can result in the formation of reactive oxygen species (ROS) that can damage cells.
In addition to the presence of an oxidizing agent, biomolecule oxidation also requires the presence of enzymes that catalyze the redox reactions involved in the process. These enzymes include oxidoreductases, which transfer electrons from one molecule to another, and dehydrogenases, which remove hydrogen atoms from molecules. The enzymes involved in biomolecule oxidation are highly specific and are regulated by a variety of factors, including the concentration of substrates and products, as well as feedback inhibition by end products.
When is Required Biomolecules Oxidation
Biomolecule oxidation is required in living organisms to produce energy that is used to fuel cellular processes. This process is necessary to sustain life and is essential for the functioning of cells, tissues, and organs.
Biomolecules, such as carbohydrates, lipids, and proteins, are oxidized in cells through a series of metabolic pathways that are coupled with the production of energy in the form of ATP. For example, glucose is oxidized through glycolysis to produce pyruvate, which is then further oxidized in the citric acid cycle to generate ATP. Similarly, lipids are oxidized through beta-oxidation to produce acetyl-CoA, which is then used in the citric acid cycle to generate ATP.
Proteins can also be oxidized, which can lead to the formation of reactive oxygen species (ROS) that can damage cells. However, cells have mechanisms in place to protect against the harmful effects of ROS, such as antioxidants.
Biomolecule oxidation is required in a variety of physiological processes, including muscle contraction, nerve conduction, and synthesis of macromolecules such as DNA and RNA. The process of biomolecule oxidation is tightly regulated to ensure that cells have the energy they need to function properly while minimizing damage from oxidative stress.
Where is Required Biomolecules Oxidation
Biomolecule oxidation occurs primarily in the mitochondria of eukaryotic cells. Mitochondria are organelles that are responsible for generating most of the energy required by cells through the process of oxidative phosphorylation.
Carbohydrates, such as glucose, are oxidized in the cytoplasm through glycolysis, but the majority of the ATP produced is generated through oxidative phosphorylation in the mitochondria. The oxidation of lipids also occurs in the mitochondria, where fatty acids are broken down through beta-oxidation to produce acetyl-CoA, which is then used in the citric acid cycle to generate ATP.
Proteins can also be oxidized, but this process occurs primarily in the cytoplasm and in organelles such as the endoplasmic reticulum and peroxisomes. However, the breakdown of amino acids from proteins ultimately feeds into the citric acid cycle in the mitochondria to generate ATP.
Overall, the mitochondria play a central role in biomolecule oxidation and energy production in eukaryotic cells. They are able to produce ATP through the oxidative phosphorylation process, which requires a constant supply of electrons from oxidized biomolecules.
How is Required Biomolecules Oxidation
Biomolecule oxidation occurs through a series of metabolic pathways that involve a variety of enzymes and cofactors. The process of biomolecule oxidation can be broadly divided into three stages: glycolysis, the citric acid cycle, and oxidative phosphorylation.
Glycolysis is the process by which glucose is broken down into pyruvate in the cytoplasm of cells. This process involves a series of enzymatic reactions that ultimately result in the production of ATP and NADH, a molecule that is used in the later stages of biomolecule oxidation.
The pyruvate produced in glycolysis is then transported into the mitochondria, where it enters the citric acid cycle. In this cycle, pyruvate is further oxidized to produce acetyl-CoA, which is then used to generate ATP through a series of enzymatic reactions that produce NADH and FADH2, molecules that are used in the final stage of biomolecule oxidation.
The final stage of biomolecule oxidation is oxidative phosphorylation, which occurs in the inner mitochondrial membrane. In this stage, the electrons carried by NADH and FADH2 are used to generate a proton gradient across the membrane, which is then used to drive the synthesis of ATP. This process is accomplished through the action of a series of enzymes called the electron transport chain, which transfers electrons from NADH and FADH2 to oxygen, the final electron acceptor.
Overall, biomolecule oxidation is a highly coordinated and regulated process that involves the action of numerous enzymes and cofactors. The process is essential for the production of ATP, which is required for a wide range of cellular processes and is necessary for life.
Structures of Biomolecules Oxidation
Biomolecule oxidation involves the oxidation of a variety of organic molecules, including carbohydrates, lipids, and proteins. The structures of these biomolecules are diverse, and their oxidation involves a series of enzymatic reactions that result in the loss of electrons and the release of energy in the form of ATP.
Carbohydrates, such as glucose, are oxidized through glycolysis, which involves the breakdown of glucose into two molecules of pyruvate. The structure of glucose is a hexose sugar with the formula C6H12O6. In glycolysis, glucose is phosphorylated to form glucose-6-phosphate, which is then oxidized to produce pyruvate. The oxidation of glucose involves the transfer of electrons to NAD+ to produce NADH, which is used to generate ATP in the later stages of biomolecule oxidation.
Lipids, such as fatty acids, are oxidized through a process called beta-oxidation, which occurs in the mitochondria. The structure of fatty acids is characterized by a long hydrocarbon chain with a carboxylic acid group at one end. During beta-oxidation, the fatty acid chain is broken down into acetyl-CoA, which is then used in the citric acid cycle to generate ATP. The oxidation of fatty acids also involves the production of NADH and FADH2, which are used to generate ATP in the final stage of biomolecule oxidation.
Proteins are composed of a variety of amino acids, which are linked together by peptide bonds. The oxidation of proteins involves the breakdown of amino acids into their constituent parts, which can then be used to generate ATP through the citric acid cycle. The oxidation of amino acids involves the removal of amino groups through a process called deamination, which produces ammonia and a carbon skeleton that can be used to generate acetyl-CoA.
Overall, the structures of biomolecules involved in oxidation are diverse, but their oxidation involves the transfer of electrons to generate ATP, which is essential for the functioning of cells and organisms.
Case Study on Biomolecules Oxidation
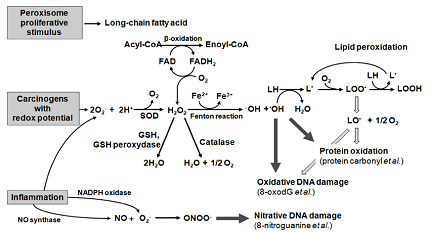
One example of biomolecule oxidation is the process of lipid peroxidation. Lipids are essential biomolecules that play a critical role in maintaining the integrity of cell membranes, and they serve as a source of energy for the body. However, under certain conditions, lipids can undergo oxidation, which can lead to the generation of reactive oxygen species (ROS) and cause damage to cells and tissues.
Lipid peroxidation is a chain reaction that occurs in the presence of ROS, such as superoxide radicals, hydroxyl radicals, and hydrogen peroxide. The reaction begins when a ROS molecule reacts with a lipid molecule, which generates a lipid radical. The lipid radical can then react with molecular oxygen to form a peroxyl radical, which can further propagate the chain reaction by reacting with other lipid molecules.
As the chain reaction progresses, it can lead to the production of various secondary products, such as malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), and isoprostanes. These products can damage cellular structures and macromolecules, including proteins, DNA, and RNA.
Lipid peroxidation can be initiated by various factors, such as exposure to environmental toxins, radiation, and inflammation. For example, exposure to ultraviolet (UV) radiation can lead to the production of ROS in the skin, which can initiate lipid peroxidation and cause skin damage. Similarly, chronic inflammation can lead to the generation of ROS by immune cells, which can contribute to the progression of various diseases, including cancer and neurodegenerative disorders.
There are various strategies to prevent or mitigate the effects of lipid peroxidation, including the consumption of antioxidants, such as vitamin E and C, which can scavenge ROS and prevent the initiation of lipid peroxidation. Additionally, certain enzymes, such as superoxide dismutase and catalase, can also neutralize ROS and protect cells from oxidative damage.
In conclusion, lipid peroxidation is a complex process that can lead to the generation of ROS and cause damage to cells and tissues. Understanding the mechanisms of lipid peroxidation and developing strategies to prevent or mitigate its effects can have significant implications for the prevention and treatment of various diseases.
White paper on Biomolecules Oxidation
Biomolecule oxidation is a complex process that can have significant implications for the maintenance of cellular homeostasis and the development of various diseases. Oxidative stress, which occurs when the production of reactive oxygen species (ROS) exceeds the capacity of antioxidant defense systems, can lead to the oxidation of biomolecules, including lipids, proteins, DNA, and RNA.
Lipid peroxidation is one example of biomolecule oxidation that can occur in cells and tissues. The process of lipid peroxidation involves the generation of ROS, which can react with lipids and initiate a chain reaction that can lead to the production of various secondary products, including malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), and isoprostanes. These products can cause damage to cellular structures and macromolecules and contribute to the development of various diseases, such as cancer, cardiovascular disease, and neurodegenerative disorders.
Protein oxidation is another example of biomolecule oxidation that can occur in cells and tissues. Protein oxidation can lead to the formation of protein carbonyls, which can alter the structure and function of proteins and contribute to the development of various diseases, such as Alzheimer’s disease, Parkinson’s disease, and diabetes.
DNA and RNA oxidation can also occur in cells and tissues, leading to the formation of DNA and RNA adducts, which can affect DNA replication, transcription, and translation and contribute to the development of various diseases, such as cancer and aging.
There are various strategies to prevent or mitigate the effects of biomolecule oxidation, including the consumption of antioxidants, such as vitamin E and C, which can scavenge ROS and prevent the initiation of biomolecule oxidation. Additionally, certain enzymes, such as superoxide dismutase and catalase, can also neutralize ROS and protect cells from oxidative damage.
In conclusion, biomolecule oxidation is a complex process that can have significant implications for cellular homeostasis and the development of various diseases. Understanding the mechanisms of biomolecule oxidation and developing strategies to prevent or mitigate its effects can have significant implications for the prevention and treatment of various diseases.
