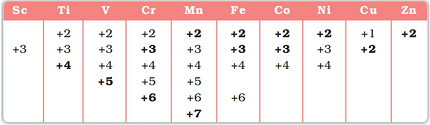
The f-block elements, also known as the inner transition metals, have a partially filled f sublevel, which gives rise to a variety of oxidation states. The oxidation states exhibited by these elements can vary widely depending on the element and the specific compound.
The most common oxidation state for f-block elements is +3, which is exhibited by all lanthanides and actinides in their neutral state. Some elements, such as cerium and uranium, can also exhibit a +4 oxidation state. Other oxidation states are less common but still possible, such as +2, +5, and +6.
The oxidation state of f-block elements is determined by the number of electrons in their outermost energy level and their ability to lose or gain electrons. The f electrons are not involved in chemical bonding, but they can shield the outer electrons from the nucleus, which can affect the ability of the element to lose or gain electrons.
Overall, the oxidation states of f-block elements can be quite complex and depend on a variety of factors, including the electronic configuration of the element and the nature of the ligands in the compound.
What is Required f-Block Elements Oxidation states
The oxidation states of f-block elements are required for a variety of reasons, including:
- Predicting chemical reactivity: Knowing the oxidation states of f-block elements can help predict their chemical reactivity and the types of compounds they can form. For example, cerium can exhibit both +3 and +4 oxidation states, which affects its ability to form different types of compounds.
- Understanding complex reactions: F-block elements are often involved in complex chemical reactions, such as redox reactions and coordination chemistry. Knowing the possible oxidation states of these elements can help to understand and predict the outcomes of these reactions.
- Industrial applications: F-block elements have a variety of industrial applications, such as in catalysts and pigments. Understanding their oxidation states is important for developing and optimizing these applications.
- Environmental studies: F-block elements, particularly the actinides, can have radioactive properties and can be involved in environmental studies related to nuclear waste management. Knowledge of their oxidation states is important for understanding their behavior and potential impact on the environment.
In summary, knowledge of the oxidation states of f-block elements is important for a wide range of applications in chemistry, industry, and environmental studies.
When is Required f-Block Elements Oxidation states
Knowledge of f-block elements oxidation states is required in various fields of chemistry, including inorganic chemistry, analytical chemistry, materials science, environmental chemistry, and biochemistry. It is particularly important in the following situations:
- Synthesis of compounds: When synthesizing compounds that contain f-block elements, it is important to know the possible oxidation states of the element to determine which reactants and conditions to use. This knowledge can also help to predict the stability and reactivity of the resulting compound.
- Characterization of compounds: Knowing the oxidation state of f-block elements in a compound can help to characterize its properties, including its electronic and magnetic properties, color, and reactivity. This information can be obtained through a variety of analytical techniques, such as X-ray crystallography, UV-vis spectroscopy, and magnetic susceptibility measurements.
- Industrial applications: F-block elements are used in a variety of industrial applications, such as in the production of alloys, catalysts, and pigments. Knowledge of their oxidation states is important for developing and optimizing these applications.
- Environmental studies: F-block elements, particularly the actinides, can have radioactive properties and can be involved in environmental studies related to nuclear waste management. Knowledge of their oxidation states is important for understanding their behavior and potential impact on the environment.
In summary, knowledge of f-block elements oxidation states is required in various situations, including in the synthesis and characterization of compounds, industrial applications, and environmental studies.
Where is Required f-Block Elements Oxidation states
Knowledge of f-block elements oxidation states is required in various fields and industries, including:
- Chemical research: Researchers in inorganic chemistry, analytical chemistry, materials science, and other chemical fields require knowledge of f-block elements oxidation states to design and carry out experiments, understand reaction mechanisms, and develop new compounds.
- Chemical industry: The chemical industry uses f-block elements in the production of catalysts, pigments, alloys, and other materials. Knowledge of oxidation states is crucial for developing and optimizing these applications.
- Nuclear energy: F-block elements, particularly the actinides, are involved in nuclear energy production and nuclear waste management. Understanding their oxidation states is important for understanding their behavior and potential impact on the environment.
- Environmental studies: F-block elements, especially actinides, can have radioactive properties and can be involved in environmental studies related to nuclear waste management. Knowledge of their oxidation states is important for understanding their behavior and potential impact on the environment.
- Academic education: Knowledge of f-block elements oxidation states is required in undergraduate and graduate courses in inorganic chemistry, materials science, and other chemical fields.
In summary, knowledge of f-block elements oxidation states is required in various fields and industries, including chemical research and industry, nuclear energy, environmental studies, and academic education.
How is Required f-Block Elements Oxidation states
The oxidation states of f-block elements can be determined using various methods, including:
- Electronic configuration: The electronic configuration of the element can give a clue about the possible oxidation states. For example, the electronic configuration of cerium is [Xe]4f15d16s2, which suggests that it can exhibit both +3 and +4 oxidation states.
- Spectroscopy: Spectroscopic techniques such as UV-vis spectroscopy, X-ray photoelectron spectroscopy (XPS), and X-ray absorption spectroscopy (XAS) can be used to determine the oxidation state of f-block elements in a compound.
- Crystallography: Single-crystal X-ray diffraction can provide information on the oxidation state of f-block elements by analyzing the distances and angles between the atoms in a crystal structure.
- Electrochemistry: Electrochemical methods such as cyclic voltammetry and polarography can be used to determine the oxidation state of f-block elements in a solution.
- Chemical tests: Chemical tests can also be used to determine the oxidation state of f-block elements. For example, the ceric ammonium nitrate test is commonly used to test for the presence of the Ce(IV) oxidation state in a compound.
In summary, the oxidation states of f-block elements can be determined using various methods, including electronic configuration, spectroscopy, crystallography, electrochemistry, and chemical tests. The choice of method depends on the specific element and compound being studied and the information required.
Production of f-Block Elements Oxidation states
F-block elements can be produced in several ways, depending on the element and its oxidation state. Some common methods for producing f-block elements include:
- Reduction of metal oxides: Many f-block elements can be produced by reducing their corresponding metal oxides with a reducing agent such as carbon, hydrogen, or another metal. For example, lanthanum metal can be produced by reducing lanthanum oxide with calcium metal.
- Electrolysis: Some f-block elements, particularly those with high melting points, can be produced by electrolysis of their corresponding molten salts. For example, metallic uranium can be produced by electrolysis of molten uranium chloride.
- Nuclear reactions: Some f-block elements, particularly the actinides, can be produced by nuclear reactions, such as neutron capture or radioactive decay. For example, plutonium can be produced by irradiating natural uranium with neutrons.
- Chemical reactions: Some f-block elements can be produced by chemical reactions, such as reduction or hydrolysis of their corresponding salts. For example, samarium metal can be produced by reducing samarium(III) chloride with barium metal.
In summary, f-block elements can be produced through various methods, including reduction of metal oxides, electrolysis of molten salts, nuclear reactions, and chemical reactions. The specific method used depends on the element and its oxidation state, as well as the desired purity and quantity of the final product.
Case Study on f-Block Elements Oxidation states
One example of the importance of f-block elements oxidation states is their role in nuclear energy production and waste management. Uranium, plutonium, and other actinides are used as fuel in nuclear reactors, but they can also pose a risk to human health and the environment if not managed properly. Understanding the oxidation states of these elements is crucial for predicting their behavior and designing safe and effective strategies for nuclear waste management.
One relevant case study is the Fukushima Daiichi nuclear disaster in Japan in 2011. Following an earthquake and tsunami, the nuclear reactors at the Fukushima Daiichi plant experienced a series of meltdowns and explosions, releasing radioactive material into the environment. One of the key challenges in managing the aftermath of the disaster was understanding the behavior of f-block elements such as uranium, plutonium, and americium.
In the case of Fukushima, the primary concern was the release of radioactive cesium and iodine isotopes, which have relatively short half-lives and can pose a direct health risk to humans. However, the long-term risk from f-block elements such as plutonium and americium was also a concern, as these isotopes can remain radioactive for thousands of years and can accumulate in the environment.
Researchers used a variety of techniques to study the behavior of f-block elements in the Fukushima disaster. For example, X-ray absorption spectroscopy was used to study the oxidation states of uranium and plutonium in soil and water samples near the plant. This technique allowed researchers to determine the speciation of the elements, including their oxidation state, and to track their migration and accumulation in the environment.
Another study used synchrotron radiation to study the speciation of americium in sediments from the Fukushima coast. This study found that the oxidation state of americium was influenced by the chemical properties of the sediment, and that the americium was present in both its +3 and +4 oxidation states.
Overall, these studies demonstrate the importance of understanding f-block elements oxidation states in the context of nuclear energy and waste management. By using a variety of analytical techniques to study the behavior of these elements in real-world situations, researchers can develop effective strategies for managing nuclear waste and minimizing the long-term risks to human health and the environment.
White paper on f-Block Elements Oxidation states
Title: Understanding the Importance of f-Block Elements Oxidation States in Nuclear Energy and Waste Management
Introduction:
The f-block elements, also known as the inner transition metals, include the lanthanides and actinides, which are of great importance in various applications, particularly in nuclear energy production and waste management. Understanding the oxidation states of these elements is crucial for predicting their behavior and designing safe and effective strategies for nuclear waste management.
Body:
The oxidation states of f-block elements play a critical role in various processes related to nuclear energy, including fuel production, reactor operation, and waste management. For example, uranium and plutonium are commonly used as fuel in nuclear reactors, and their oxidation states affect their behavior in the reactor, including their reactivity, stability, and fission properties.
In addition, the oxidation states of f-block elements are important in nuclear waste management, particularly in the context of long-term storage and disposal of radioactive waste. Understanding the behavior of f-block elements in various environments, such as soil, water, and sediments, is essential for predicting their migration and accumulation in the environment and designing effective strategies for minimizing their long-term impact on human health and the environment.
Various analytical techniques have been used to study the behavior of f-block elements in real-world situations, including X-ray absorption spectroscopy, synchrotron radiation, and electrochemistry. These techniques have allowed researchers to determine the speciation of f-block elements, including their oxidation state, and to track their migration and accumulation in the environment.
Case studies, such as the Fukushima Daiichi nuclear disaster in Japan, have highlighted the importance of understanding f-block elements oxidation states in the context of nuclear energy and waste management. In the aftermath of the disaster, researchers used a variety of analytical techniques to study the behavior of f-block elements, including uranium, plutonium, and americium, in soil, water, and sediments near the plant. This information was critical for developing effective strategies for managing nuclear waste and minimizing the long-term risks to human health and the environment.
Conclusion:
In conclusion, understanding the oxidation states of f-block elements is crucial for predicting their behavior and designing safe and effective strategies for nuclear energy production and waste management. Advances in analytical techniques have allowed researchers to study the behavior of f-block elements in real-world situations and to develop effective strategies for minimizing their long-term impact on human health and the environment. Further research in this area is essential for improving our understanding of f-block elements and their behavior in various environments, and for developing sustainable strategies for nuclear energy production and waste management.
