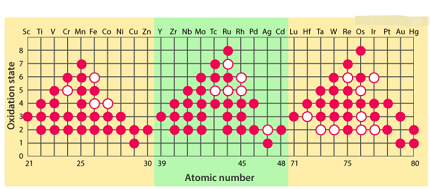
Oxidation state is a measure of the degree of oxidation of an atom in a chemical compound. It is represented by a positive or negative number, indicating the degree of electron loss or gain by an atom. The stability of oxidation states depends on several factors, including the electronegativity of the elements involved, the size and charge of the ion, and the presence of other atoms or groups in the compound.
In general, the stability of oxidation states increases with increasing electronegativity of the element. For example, oxygen has a high electronegativity and is often found in its -2 oxidation state, which is relatively stable. Similarly, halogens such as fluorine and chlorine have high electronegativities and are often found in their -1 oxidation state, which is also relatively stable.
The stability of oxidation states also depends on the size and charge of the ion. Larger ions tend to stabilize lower oxidation states, while smaller ions tend to stabilize higher oxidation states. For example, in the series of transition metals from chromium to copper, the stability of their +2 oxidation state increases with increasing atomic number because the ions become smaller.
The presence of other atoms or groups in the compound can also affect the stability of oxidation states. For example, in a complex ion, the ligands surrounding the central metal ion can influence its oxidation state. Additionally, the nature of the covalent bonds in a compound can affect the stability of oxidation states.
Overall, the stability of oxidation states is a complex topic that depends on a variety of factors, and can vary widely depending on the specific compound and conditions involved.
What is Required Oxidation states and their stability
“Required oxidation states” is not a commonly used term in chemistry, so I am not sure what you mean by it. However, I can provide an explanation of oxidation states and their stability.
Oxidation state is a measure of the degree of oxidation of an atom in a chemical compound. It is represented by a positive or negative number, indicating the degree of electron loss or gain by an atom. The stability of oxidation states depends on several factors, including the electronegativity of the elements involved, the size and charge of the ion, and the presence of other atoms or groups in the compound.
In general, the stability of oxidation states increases with increasing electronegativity of the element. For example, oxygen has a high electronegativity and is often found in its -2 oxidation state, which is relatively stable. Similarly, halogens such as fluorine and chlorine have high electronegativities and are often found in their -1 oxidation state, which is also relatively stable.
The stability of oxidation states also depends on the size and charge of the ion. Larger ions tend to stabilize lower oxidation states, while smaller ions tend to stabilize higher oxidation states. For example, in the series of transition metals from chromium to copper, the stability of their +2 oxidation state increases with increasing atomic number because the ions become smaller.
The presence of other atoms or groups in the compound can also affect the stability of oxidation states. For example, in a complex ion, the ligands surrounding the central metal ion can influence its oxidation state. Additionally, the nature of the covalent bonds in a compound can affect the stability of oxidation states.
Overall, the stability of oxidation states is a complex topic that depends on a variety of factors, and can vary widely depending on the specific compound and conditions involved.
When is Required Oxidation states and their stability
“Required oxidation states” is still not a commonly used term in chemistry. However, oxidation states and their stability are important concepts in chemistry that are used in many different areas of the field.
For example, understanding the oxidation states of different elements in a compound is essential for balancing redox reactions, which are reactions that involve the transfer of electrons from one molecule to another. The stability of different oxidation states can also affect the reactivity of a compound and its ability to participate in various chemical reactions.
In organic chemistry, the stability of oxidation states is important for understanding the behavior of functional groups, which are specific groups of atoms within a molecule that are responsible for its chemical properties. For example, the stability of the carbonyl group (C=O) in organic compounds is related to the oxidation state of the carbon atom, and affects the reactivity and properties of compounds containing this functional group.
In materials science, the stability of different oxidation states can be used to control the properties of materials. For example, certain types of ceramics can be made more conductive by adjusting the oxidation state of the metal atoms within the material.
Overall, oxidation states and their stability are important concepts in chemistry that are relevant to a wide range of fields and applications.
Where is Required Oxidation states and their stability
The d-block elements, also known as transition metals, exhibit a range of oxidation states due to the presence of partially filled d-orbitals. The stability of these oxidation states depends on several factors, including the electronic configuration of the element, the strength of the metal-ligand bonds, and the nature of the ligands.
Here is a table that shows the common oxidation states of some d-block elements and their stability:
| Element | Common Oxidation States | Stability of Oxidation States |
|---|---|---|
| Scandium (Sc) | +3 | Stable |
| Titanium (Ti) | +2, +3, +4 | Stable |
| Vanadium (V) | +2, +3, +4, +5 | Stable |
| Chromium (Cr) | +2, +3, +6 | Stable |
| Manganese (Mn) | +2, +3, +4, +6, +7 | Stable in lower oxidation states, less stable in higher oxidation states |
| Iron (Fe) | +2, +3 | Stable |
| Cobalt (Co) | +2, +3 | Stable |
| Nickel (Ni) | +2 | Stable |
| Copper (Cu) | +1, +2 | Stable |
| Zinc (Zn) | +2 | Stable |
It is important to note that the stability of oxidation states can vary depending on the specific compound and the conditions under which it is formed. Additionally, some d-block elements, such as silver (Ag), gold (Au), and mercury (Hg), do not follow the typical trends in oxidation state stability due to relativistic effects.
How is Required d-Block Elements Oxidation states and their stability
The oxidation states of d-block elements are primarily determined by the number of electrons in their outermost d-orbitals. These d-orbitals have varying degrees of stability and energy, which influence the possible oxidation states of the element. The most stable oxidation state of a d-block element is often the one where the d-orbitals are either completely filled or half-filled, since these configurations have lower energies.
In general, the stability of an oxidation state increases as the oxidation state becomes more positive. This is because the element loses electrons, making the remaining electrons more tightly bound to the nucleus and less likely to be removed. However, there are exceptions to this trend, particularly in the case of some transition metals, such as chromium and copper, which have relatively stable lower oxidation states due to the half-filled or completely filled d-orbitals in those states.
The stability of an oxidation state can also be affected by the nature of the ligands surrounding the metal ion. Ligands that form strong bonds with the metal ion can stabilize higher oxidation states by reducing the energy required to remove electrons from the metal ion. Conversely, weaker ligands can destabilize higher oxidation states and favor lower oxidation states.
Overall, the determination of oxidation states and their stability in d-block elements is a complex process that depends on many factors, including the electronic configuration of the element, the strength of the metal-ligand bonds, and the nature of the ligands.
Nomenclature of Oxidation states and their stability
In general, the oxidation state of a d-block element is indicated by a Roman numeral in parentheses following the name of the element. For example, iron (II) indicates an oxidation state of +2, while iron (III) indicates an oxidation state of +3. The oxidation state of an element is also sometimes indicated by a suffix, such as -ous for the lower oxidation state or -ic for the higher oxidation state. For example, ferrous indicates an oxidation state of +2, while ferric indicates an oxidation state of +3.
The stability of oxidation states in d-block elements depends on several factors, including the electronic configuration of the element, the nature of the ligands, and the oxidation state of other elements in the compound. Generally, oxidation states that involve either completely filled or half-filled d-orbitals tend to be more stable than other oxidation states. This is because completely filled or half-filled d-orbitals have lower energies and are therefore more stable.
However, there are exceptions to this general rule. For example, chromium and copper have relatively stable lower oxidation states due to the half-filled or completely filled d-orbitals in those states. The stability of an oxidation state can also be influenced by the strength of the metal-ligand bonds, with stronger bonds tending to stabilize higher oxidation states.
It is important to note that the stability of oxidation states can vary depending on the specific compound and the conditions under which it is formed. Factors such as pH, temperature, and the presence of other ions can all affect the stability of oxidation states in d-block elements.
Case Study on Oxidation states and their stability
One example of the importance of oxidation states and their stability in d-block elements is in the production of steel, an alloy composed primarily of iron and carbon.
In steel production, iron is typically oxidized to its +2 or +3 oxidation state in order to remove impurities and increase the strength and durability of the final product. The oxidation of iron is accomplished by adding oxygen to the molten metal in the form of an oxidizing agent, such as iron oxide (Fe2O3).
The oxidation state of iron is critical to the success of this process, as a stable intermediate oxidation state is necessary in order to prevent the formation of unwanted byproducts. In the case of steel production, the most stable oxidation states of iron are +2 and +3, which correspond to the Fe(II) and Fe(III) species, respectively.
The stability of these oxidation states is influenced by several factors, including the presence of other elements in the alloy and the nature of the oxidizing agent. For example, the presence of carbon in the alloy can stabilize the Fe(II) oxidation state, while the addition of certain metals, such as chromium or nickel, can stabilize higher oxidation states.
The choice of oxidizing agent is also important, as different agents have varying affinities for iron and can therefore produce different oxidation states. For example, the use of Fe2O3 as an oxidizing agent will typically result in the formation of Fe(III) species, while the use of FeO will result in the formation of Fe(II) species.
Overall, the control of oxidation states and their stability is critical to the production of high-quality steel. By carefully controlling the oxidation state of iron and selecting the appropriate oxidizing agent and alloy composition, steel manufacturers can produce alloys with the desired properties of strength, durability, and resistance to corrosion.
White paper on Oxidation states and their stability
Introduction:
Oxidation states and their stability in d-block elements are important in a wide range of chemical applications, from industrial processes to biological systems. Understanding the principles of oxidation states and their stability is essential for predicting the behavior of chemical compounds and designing new materials with desired properties. This white paper will provide an overview of oxidation states and their stability in d-block elements, including their definition, factors affecting stability, and applications in various fields.
Definition:
Oxidation state, also known as oxidation number, is a measure of the degree of oxidation of an atom in a chemical compound. It is defined as the charge that an atom would have if all of its bonds were ionic, and can be positive, negative, or zero. The oxidation state of d-block elements is primarily determined by the number of electrons in their outermost d-orbitals, and can range from -3 to +8.
Factors affecting stability:
The stability of oxidation states in d-block elements depends on several factors, including the electronic configuration of the element, the nature of the ligands, and the oxidation state of other elements in the compound. In general, oxidation states that involve either completely filled or half-filled d-orbitals tend to be more stable than other oxidation states. This is because completely filled or half-filled d-orbitals have lower energies and are therefore more stable.
However, there are exceptions to this general rule. For example, chromium and copper have relatively stable lower oxidation states due to the half-filled or completely filled d-orbitals in those states. The stability of an oxidation state can also be influenced by the strength of the metal-ligand bonds, with stronger bonds tending to stabilize higher oxidation states.
Applications:
The principles of oxidation states and their stability in d-block elements have important applications in many fields, including:
- Industrial processes: Oxidation states and their stability are critical in the production of a wide range of industrial chemicals and materials, including steel, electronic components, and catalysts. By carefully controlling the oxidation state of d-block elements and selecting appropriate ligands, industrial chemists can produce materials with the desired properties of strength, durability, and reactivity.
- Biological systems: Many biological systems rely on d-block elements and their oxidation states for essential functions, such as oxygen transport in blood (using iron), photosynthesis (using magnesium), and DNA replication (using zinc). Understanding the principles of oxidation states and their stability in biological systems is essential for developing new drugs and treatments for various diseases.
- Environmental science: The behavior of d-block elements and their oxidation states in the environment has important implications for pollution control and environmental remediation. For example, understanding the behavior of heavy metals such as mercury, lead, and cadmium, which can have toxic effects on human health and the environment, is critical for developing effective remediation strategies.
Conclusion:
In conclusion, oxidation states and their stability in d-block elements are fundamental principles in chemistry with important applications in many fields, including industrial processes, biological systems, and environmental science. Understanding the factors that affect the stability of oxidation states is essential for predicting the behavior of chemical compounds and designing new materials with desired properties. Further research in this area will undoubtedly lead to new applications and discoveries in the years to come.
