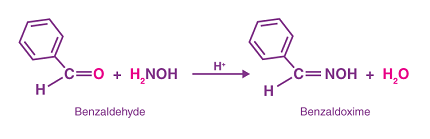
An oxime is a chemical compound that contains a nitrogen atom connected to a carbon atom via a double bond (C=N-OH). Oximes are typically formed by the reaction of aldehydes or ketones with hydroxylamine. They are important intermediates in organic synthesis and are used in a variety of applications, including as reagents in analytical chemistry, as stabilizers for polymers, and as chelating agents in metal extraction.
Oximes have a characteristic absorption band in their infrared spectrum, which allows them to be easily identified. They also have the ability to form complexes with metal ions, making them useful in the extraction and separation of metals. Additionally, oximes can be used in the treatment of nerve agent poisoning, as they can react with certain nerve agents and prevent them from binding to acetylcholinesterase, which is necessary for nerve function.
What is Required Aldehydes and Ketones Oxime
To synthesize an oxime, an aldehyde or ketone is required as the starting material. The carbonyl group in the aldehyde or ketone is first converted to an oxime group by reaction with hydroxylamine, which is an amine containing an -OH group.
The reaction typically takes place in the presence of an acid catalyst, such as hydrochloric acid or sulfuric acid, and involves the addition of hydroxylamine to the carbonyl group of the aldehyde or ketone to form an intermediate imine. This imine then undergoes a nucleophilic attack by water to form the final oxime product.
The general reaction scheme can be represented as follows:
RCHO + NH2OH → RCH=N-OH → RCH(NO)H
or
R2C=O + NH2OH → R2C=N-OH → R2C(NO)H
where R represents an alkyl, aryl, or other organic group.
When is Required Aldehydes and Ketones Oxime
Oximes can be synthesized from aldehydes and ketones in a variety of applications in organic chemistry. Here are a few examples:
- Protection of carbonyl groups: Oximes can be used to protect carbonyl groups in aldehydes and ketones. The oxime group can be easily removed under mild conditions, which allows for the selective deprotection of other functional groups.
- Synthesis of hydrazones: Hydrazones are compounds that contain a carbon-nitrogen double bond and a nitrogen-nitrogen double bond. They are synthesized by the reaction of an aldehyde or ketone with hydrazine. Oximes can be used as intermediates in the synthesis of hydrazones.
- Analysis of aldehydes and ketones: Oximes can be used as reagents in the qualitative and quantitative analysis of aldehydes and ketones. The formation of an oxime can be used as a test for the presence of a carbonyl group in a compound.
- Extraction of metal ions: Oximes can be used as chelating agents in the extraction and separation of metal ions. The oxime group can bind to metal ions, which allows for their separation from other ions in a mixture.
- Treatment of nerve agent poisoning: Oximes can be used in the treatment of nerve agent poisoning. The oxime group can react with certain nerve agents and prevent them from binding to acetylcholinesterase, which is necessary for nerve function.
Where is Required Aldehydes and Ketones Oxime
Aldehydes and ketones can be found in a variety of natural and synthetic compounds. They are commonly used in the synthesis of pharmaceuticals, fragrances, and flavorings, as well as in the production of polymers and plastics.
Oximes, which are synthesized from aldehydes and ketones, are used in a wide range of applications in organic chemistry, analytical chemistry, and biochemistry. They are used in the protection of carbonyl groups, the synthesis of hydrazones, and the extraction of metal ions.
In addition, oximes have applications in the treatment of nerve agent poisoning, which is a concern in military and civilian settings. Oximes are used to treat individuals who have been exposed to nerve agents, such as sarin or VX, by reactivating acetylcholinesterase and preventing the toxic effects of the nerve agent.
How is Required Aldehydes and Ketones Oxime
The synthesis of oximes from aldehydes and ketones involves the reaction of the carbonyl group with hydroxylamine to form the corresponding oxime. The reaction can be catalyzed by an acid, such as hydrochloric acid or sulfuric acid, and typically requires mild reaction conditions.
Here are the general steps involved in the synthesis of an oxime:
- Preparation of hydroxylamine: Hydroxylamine can be prepared by the reduction of nitrous acid or nitrite salts using a reducing agent such as sodium sulfite or sodium bisulfite.
- Reaction of hydroxylamine with the aldehyde or ketone: The hydroxylamine is added to the aldehyde or ketone in the presence of an acid catalyst. The reaction results in the formation of an imine intermediate.
- Addition of water: The imine intermediate is hydrolyzed with the addition of water to form the final oxime product.
The reaction can be represented by the following equation:
RCHO/R2C=O + NH2OH → RCH=N-OH/R2C=N-OH → RCH(NO)H/R2C(NO)H
The product, an oxime, is a stable compound that can be purified by standard methods such as distillation, crystallization, or chromatography.
Production of Aldehydes and Ketones Oxime
Aldehydes and ketones oximes can be produced through a variety of methods, including:
- Acid-catalyzed reaction: This method involves the addition of hydroxylamine to the carbonyl group of an aldehyde or ketone in the presence of an acid catalyst, such as hydrochloric acid or sulfuric acid. The reaction produces an oxime as the final product.
- Base-catalyzed reaction: In this method, hydroxylamine is added to the carbonyl group of an aldehyde or ketone in the presence of a base catalyst, such as sodium hydroxide or potassium hydroxide. The reaction produces an oxime as the final product.
- Microwave-assisted reaction: Microwave irradiation can be used to promote the reaction between an aldehyde or ketone and hydroxylamine. The reaction is typically carried out in the presence of an acid catalyst and produces an oxime as the final product.
- Enzymatic synthesis: Certain enzymes, such as hydroxylamine oxidoreductase, can catalyze the reaction between an aldehyde or ketone and hydroxylamine to produce an oxime.
The choice of method depends on the specific application and desired outcome, as well as the starting materials available. The acid-catalyzed and base-catalyzed methods are the most commonly used methods for the synthesis of oximes from aldehydes and ketones.
Case Study on Aldehydes and Ketones Oxime
One example of the use of aldehydes and ketones oximes is in the development of antidotes for organophosphate nerve agent poisoning. Organophosphate nerve agents, such as sarin and VX, inhibit the activity of acetylcholinesterase, an enzyme that is necessary for normal nerve function. The resulting accumulation of acetylcholine can lead to overstimulation of the nervous system and can be lethal if left untreated.
Oximes can be used as antidotes for organophosphate poisoning by reactivating acetylcholinesterase and allowing it to break down the excess acetylcholine. The oxime works by binding to the organophosphate compound and displacing it from the enzyme, thereby restoring its activity.
One example of an oxime-based antidote is pralidoxime chloride (2-PAM), which is used in the treatment of nerve agent poisoning. 2-PAM is synthesized by reacting pyridine-2-aldehyde with methyl ethyl ketoxime in the presence of hydrochloric acid. The resulting oxime is then reacted with an aqueous solution of sodium hydroxide to produce the final product, pralidoxime chloride.
The mechanism of action of 2-PAM involves the nucleophilic attack of the oxime group on the phosphorus atom of the organophosphate compound, which results in the formation of a phosphonate-oxime adduct. The adduct is then hydrolyzed by water to release the oxime and regenerate the active enzyme.
The use of oxime-based antidotes in the treatment of nerve agent poisoning has been the subject of ongoing research and development. The development of new and more effective oxime-based antidotes, as well as the optimization of dosing and delivery methods, is an area of active investigation.
White paper on Aldehydes and Ketones Oxime
Introduction:
Aldehydes and ketones are organic compounds that contain a carbonyl group (-C=O) and are widely used in various industries. Oximes are organic compounds that are formed by the reaction of hydroxylamine with a carbonyl group. Aldehydes and ketones oximes have various applications in different fields, including pharmaceuticals, agriculture, and chemical industries.
This white paper will discuss the properties and applications of aldehydes and ketones oximes.
Properties:
Aldehydes and ketones oximes are generally white to yellow crystalline solids with a characteristic odor. They are soluble in polar solvents, such as water, ethanol, and methanol, but are insoluble in non-polar solvents, such as hexane and chloroform. The melting and boiling points of oximes depend on the specific compound, but are generally higher than those of the corresponding aldehydes and ketones.
Applications:
- Pharmaceutical industry: Aldehydes and ketones oximes are used as starting materials for the synthesis of various pharmaceuticals, including antituberculosis drugs, antidepressants, and anticonvulsants. They are also used in the development of antidotes for organophosphate nerve agent poisoning, as discussed in the case study above.
- Agriculture: Oximes are used as plant growth regulators, insecticides, and herbicides. For example, methoxyiminoacetate (MOIA) is an oxime derivative that is used as a herbicide.
- Chemical industry: Aldehydes and ketones oximes are used in the production of various chemicals, including solvents, plasticizers, and surfactants. They are also used in the synthesis of specialty polymers and resins.
- Analytical chemistry: Oximes are used as derivatizing agents in chromatography and spectrophotometry to enhance the detection and separation of various compounds, such as amino acids and sugars.
Conclusion:
Aldehydes and ketones oximes are versatile organic compounds that have a wide range of applications in various industries. They are important intermediates in the synthesis of pharmaceuticals and agrochemicals, and are used in the production of various chemicals and materials. The properties and applications of aldehydes and ketones oximes make them an important class of compounds in modern chemistry.
