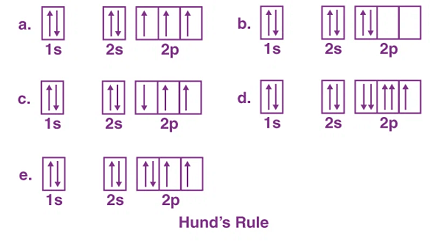
Pauli’s exclusion principle and Hund’s rule are both fundamental principles of quantum mechanics that describe the behavior of electrons in atoms.
Pauli’s exclusion principle states that no two electrons in an atom can have the same set of quantum numbers. In other words, each electron in an atom must have a unique combination of values for its energy level, orbital shape, and orientation. This means that electrons in the same orbital must have opposite spins, which allows them to occupy the same space without repelling each other.
Hund’s rule, on the other hand, states that when multiple orbitals of the same energy level are available, electrons will occupy separate orbitals with parallel spins before they pair up in the same orbital. This means that in a partially filled subshell, electrons will first occupy separate orbitals with the same energy level and spin direction, rather than pairing up in the same orbital with opposite spins.
Together, Pauli’s exclusion principle and Hund’s rule help to explain the electronic configuration of atoms and the behavior of chemical reactions. These principles provide a basis for understanding the structure and properties of materials, as well as the behavior of electrons in a wide range of physical and chemical phenomena.
What is Required Pauli’s exclusion principle and Hund’s rule
Pauli’s exclusion principle and Hund’s rule are both required in quantum mechanics to explain the behavior of electrons in atoms and molecules.
Pauli’s exclusion principle is necessary to explain the electronic configuration of atoms, and why electrons don’t collapse into the same energy state. It states that no two electrons can have the same set of quantum numbers, which means that electrons in the same orbital must have opposite spins to avoid being in the same energy state.
Hund’s rule is necessary to explain the arrangement of electrons in partially filled subshells, where there are multiple orbitals of the same energy level. It states that electrons will occupy separate orbitals with parallel spins before they pair up in the same orbital, which results in a lower energy configuration.
Together, these principles help to explain the electronic structure of atoms and molecules, and provide a foundation for understanding chemical reactions and other physical phenomena. Without these principles, our understanding of the behavior of electrons in atoms and molecules would be incomplete.
Who is Required Pauli’s exclusion principle and Hund’s rule
Pauli’s exclusion principle and Hund’s rule were proposed by two different scientists:
- Pauli’s exclusion principle was first proposed by the Austrian physicist Wolfgang Pauli in 1925. Pauli’s principle states that no two electrons in an atom can have the same set of quantum numbers, which means that electrons in the same orbital must have opposite spins to avoid being in the same energy state.
- Hund’s rule was proposed by the German physicist Friedrich Hund in 1927. Hund’s rule states that when multiple orbitals of the same energy level are available, electrons will occupy separate orbitals with parallel spins before they pair up in the same orbital. This results in a lower energy configuration and more stable electronic arrangement.
Both of these principles are fundamental to our understanding of the behavior of electrons in atoms and molecules, and have wide-ranging applications in chemistry, physics, and materials science.
When is Required Pauli’s exclusion principle and Hund’s rule
Pauli’s exclusion principle and Hund’s rule are required whenever we need to describe the electronic configuration and behavior of electrons in atoms and molecules. These principles are especially important in the following areas:
- Atomic and molecular structure: Pauli’s exclusion principle and Hund’s rule help us to understand the arrangement of electrons in atoms and molecules. They are essential in predicting the chemical properties of elements and compounds, and in understanding how atoms and molecules interact with each other.
- Chemical bonding: Understanding the electronic configuration of atoms and molecules is crucial in understanding chemical bonding. Pauli’s exclusion principle and Hund’s rule help us to understand how electrons participate in bonding and how the resulting molecules are formed.
- Material science: Pauli’s exclusion principle and Hund’s rule are fundamental to our understanding of the electronic properties of materials. They help us to predict and control the electronic behavior of materials, which is important in the design of new materials for various applications.
- Quantum mechanics: Pauli’s exclusion principle and Hund’s rule are fundamental principles in quantum mechanics, and they are essential in developing theories and models to describe the behavior of electrons in atoms and molecules.
Overall, Pauli’s exclusion principle and Hund’s rule are required in a wide range of scientific disciplines where the behavior of electrons is important.
Where is Required Pauli’s exclusion principle and Hund’s rule
Pauli’s exclusion principle and Hund’s rule are required in many different areas of science where the behavior of electrons is important. These principles are used in a variety of fields and applications, including:
- Atomic and molecular physics: Pauli’s exclusion principle and Hund’s rule are fundamental principles in the study of atomic and molecular physics. They are used to predict the electronic configuration of atoms and molecules, and to understand the interactions between electrons in these systems.
- Chemistry: Pauli’s exclusion principle and Hund’s rule are essential in the study of chemical bonding and reaction mechanisms. They are used to understand the electronic structure of molecules, and to predict how different atoms will bond with each other.
- Materials science: Pauli’s exclusion principle and Hund’s rule are important in the study of materials science, where they are used to understand the electronic properties of materials. This understanding is crucial in designing and engineering new materials for various applications.
- Quantum mechanics: Pauli’s exclusion principle and Hund’s rule are fundamental principles in quantum mechanics, which is a field that studies the behavior of matter and energy at the smallest scales. These principles are essential in developing models and theories to describe the behavior of electrons in atoms and molecules.
Overall, Pauli’s exclusion principle and Hund’s rule are required in many different areas of science where the behavior of electrons is important, including physics, chemistry, materials science, and quantum mechanics.
How is Required Pauli’s exclusion principle and Hund’s rule
Pauli’s exclusion principle and Hund’s rule describe the behavior of electrons in atoms and molecules, and they are essential in understanding a variety of phenomena related to electronic configuration and chemical bonding.
Pauli’s exclusion principle states that no two electrons in an atom can have the same set of quantum numbers, which means that electrons in the same orbital must have opposite spins to avoid being in the same energy state. This principle helps to explain the stability of atoms and prevents electrons from collapsing into the same energy state, which would result in an unstable configuration.
Hund’s rule states that electrons will occupy separate orbitals with parallel spins before they pair up in the same orbital. This results in a lower energy configuration and more stable electronic arrangement. This principle helps to explain the arrangement of electrons in partially filled subshells, where there are multiple orbitals of the same energy level.
Together, these principles help to explain the electronic structure of atoms and molecules, and provide a foundation for understanding chemical reactions and other physical phenomena. They are used to predict and understand the behavior of electrons in a variety of systems, from simple atoms to complex molecules and materials.
In practice, scientists use these principles to predict the electronic configuration of atoms and molecules, to design and engineer new materials with specific electronic properties, and to develop models and theories to explain a wide range of physical and chemical phenomena.
Case Study on Pauli’s exclusion principle and Hund’s rule
One example of how Pauli’s exclusion principle and Hund’s rule can be used in a case study is in the study of the electronic structure of transition metal complexes.
Transition metal complexes are molecules that contain a transition metal atom surrounded by ligands, which are molecules or ions that bind to the metal atom through coordination bonds. These complexes have important applications in areas such as catalysis, materials science, and biochemistry.
To understand the electronic structure of transition metal complexes, it is necessary to apply Pauli’s exclusion principle and Hund’s rule. The electrons in these complexes are organized into energy levels or orbitals, which are characterized by their quantum numbers.
Pauli’s exclusion principle states that no two electrons in an atom or molecule can have the same set of quantum numbers, which means that electrons in the same orbital must have opposite spins. This principle is important in transition metal complexes because the metal atom can have multiple electrons in the same energy level or orbital. For example, in a complex containing a d-block transition metal, the five d-orbitals can each hold up to two electrons with opposite spins.
Hund’s rule states that electrons will occupy separate orbitals with parallel spins before they pair up in the same orbital. This principle is important in transition metal complexes because it determines the order in which the d-orbitals are filled. For example, if a transition metal complex has four d-electrons, they will occupy separate orbitals with parallel spins before pairing up in the same orbital. This results in a lower energy configuration and a more stable electronic arrangement.
By applying Pauli’s exclusion principle and Hund’s rule, scientists can predict the electronic configuration of transition metal complexes and understand their chemical and physical properties. This understanding is crucial in designing and engineering new transition metal complexes for various applications, such as catalysis, energy storage, and drug design.
White paper on Pauli’s exclusion principle and Hund’s rule
Introduction:
Pauli’s exclusion principle and Hund’s rule are two fundamental principles of quantum mechanics that describe the behavior of electrons in atoms and molecules. These principles are essential in understanding the electronic structure of matter and are applied in many fields of science, including physics, chemistry, materials science, and engineering.
In this white paper, we will discuss Pauli’s exclusion principle and Hund’s rule in detail, explaining their origin, their practical applications, and their significance in modern science.
Pauli’s exclusion principle:
Pauli’s exclusion principle was first introduced by Austrian physicist Wolfgang Pauli in 1925. The principle states that no two electrons in an atom or molecule can have the same set of quantum numbers. This means that if two electrons occupy the same orbital, they must have opposite spins.
The quantum numbers that describe an electron’s state include its energy level, angular momentum, magnetic moment, and spin. According to Pauli’s exclusion principle, the maximum number of electrons that can occupy an orbital is two: one with spin-up and one with spin-down.
The principle is important in understanding the stability of atoms and molecules. Without Pauli’s exclusion principle, electrons would collapse into the lowest energy state, resulting in an unstable configuration. The principle also explains the observed electron configurations of atoms, which are essential in predicting chemical properties and reactions.
Hund’s rule:
Hund’s rule, named after German physicist Friedrich Hund, describes the way electrons fill orbitals in atoms and molecules. The rule states that electrons will occupy separate orbitals with parallel spins before they pair up in the same orbital.
For example, if a d-subshell has five electrons, the electrons will occupy separate orbitals with parallel spins before pairing up in the same orbital. This configuration results in a lower energy state and a more stable electronic arrangement.
Hund’s rule is important in understanding the electronic structure of atoms and molecules. It helps to predict the arrangement of electrons in partially filled subshells, which are common in transition metal complexes and other systems. The rule is also used in the design and engineering of new materials with specific electronic properties.
Applications of Pauli’s exclusion principle and Hund’s rule:
Pauli’s exclusion principle and Hund’s rule have numerous applications in modern science and technology. They are used in a wide range of fields, including:
- Atomic and molecular physics: The principles are essential in predicting the electronic configuration and behavior of atoms and molecules.
- Chemistry: The principles are important in understanding chemical bonding and reaction mechanisms, which are essential in drug design, materials science, and catalysis.
- Materials science: The principles are used to understand the electronic properties of materials, which is crucial in designing new materials for energy storage, electronic devices, and other applications.
- Quantum mechanics: The principles are fundamental in developing models and theories to describe the behavior of electrons in atoms and molecules.
Conclusion:
Pauli’s exclusion principle and Hund’s rule are two fundamental principles of quantum mechanics that describe the behavior of electrons in atoms and molecules. These principles are essential in understanding the electronic structure of matter and have numerous applications in modern science and technology. By applying these principles, scientists can predict the electronic configuration of atoms and molecules, design and engineer new materials with specific electronic properties, and develop models and theories to explain a wide range of physical and chemical phenomena.
