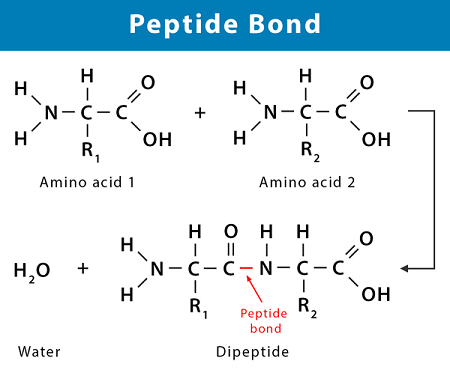
Peptide linkage, also known as amide linkage, is the covalent bond that forms between the carboxyl group (–COOH) of one amino acid and the amino group (–NH2) of another amino acid during protein synthesis. The bond is formed through a dehydration synthesis reaction, which involves the removal of a water molecule.
The resulting bond between the two amino acids is called a peptide bond, and the sequence of peptide bonds between amino acids forms the backbone of a protein. The peptide bond has a partial double bond character due to resonance between the carbonyl group and the nitrogen atom, which gives it a rigid and planar structure.
The peptide bond plays a crucial role in determining the three-dimensional structure and function of proteins. The specific sequence of amino acids and the resulting peptide bonds between them determine the folding of the protein into its functional three-dimensional shape.
What is Required Biomolecules Peptide linkage
The required biomolecules for the formation of a peptide linkage are amino acids. Amino acids are organic molecules that contain an amino group (-NH2), a carboxyl group (-COOH), and a side chain (-R). During protein synthesis, the carboxyl group of one amino acid reacts with the amino group of another amino acid, forming a peptide bond and releasing a molecule of water. This process is called dehydration synthesis or condensation reaction.
The amino acid sequence of a protein is determined by the genetic code, which specifies the order of amino acids in a protein. The specific sequence of amino acids in a protein, as well as the peptide bonds between them, determines the protein’s unique three-dimensional structure and its function in the body.
Therefore, the formation of peptide bonds between amino acids is a crucial step in the synthesis of proteins, which are essential biomolecules for various biological processes in the body.
When is Required Biomolecules Peptide linkage
Peptide linkage is required during the process of protein synthesis. Proteins are large, complex biomolecules that are essential for many biological processes, including enzyme catalysis, cell signaling, and structural support. Proteins are made up of long chains of amino acids that are linked together by peptide bonds.
During protein synthesis, the process of translation converts the genetic information in mRNA (messenger RNA) into a sequence of amino acids that make up the protein. The ribosome, which is a complex molecular machine, reads the mRNA sequence and assembles the protein by linking together amino acids through peptide bonds.
Each peptide bond is formed between the carboxyl group of one amino acid and the amino group of another amino acid. This process occurs repeatedly, and the resulting sequence of amino acids and peptide bonds determines the structure and function of the protein.
Therefore, peptide linkage is required during the process of protein synthesis to form the backbone of proteins and to provide the specific sequence of amino acids that are necessary for the protein’s unique structure and function.
Where is Required Biomolecules Peptide linkage
Peptide linkage is primarily found in proteins, which are one of the four major classes of biomolecules found in living organisms. Proteins are found in every cell of the body and are involved in a wide range of biological functions, including enzyme catalysis, cell signaling, structural support, transport of molecules, and defense against pathogens.
Peptide linkage forms the backbone of proteins and is responsible for the linear sequence of amino acids that make up the protein. The specific sequence of amino acids and the resulting peptide bonds determine the three-dimensional structure of the protein, which is critical for its function.
Peptide linkage is also found in other biomolecules, such as peptides and polypeptides, which are smaller chains of amino acids. Peptides are typically composed of between 2 and 50 amino acids, while polypeptides can contain hundreds or thousands of amino acids.
Overall, peptide linkage is a fundamental chemical bond that is essential for the formation of proteins and other biomolecules that are critical for life.
How is Required Biomolecules Peptide linkage
Peptide linkage is formed through a condensation reaction, also known as a dehydration synthesis reaction, between the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of another amino acid. During this reaction, a molecule of water is released, and a peptide bond is formed between the two amino acids.
The process of peptide bond formation occurs during protein synthesis, which is a complex biological process that occurs in all living organisms. Protein synthesis involves two main stages: transcription and translation.
During transcription, the genetic information in DNA is transcribed into mRNA (messenger RNA), which carries the genetic code to the ribosome.
During translation, the ribosome reads the mRNA sequence and assembles the protein by linking together amino acids through peptide bonds. Each amino acid is brought to the ribosome by a transfer RNA (tRNA) molecule, and the ribosome links the amino acids together in the correct order, forming a polypeptide chain.
As the polypeptide chain grows, the peptide bonds between the amino acids are formed by the condensation reaction between the carboxyl group of one amino acid and the amino group of the next amino acid. This process continues until the entire protein sequence is assembled, and the protein is folded into its three-dimensional structure.
Overall, peptide linkage is formed through a complex series of biochemical reactions that occur during protein synthesis, which is a critical process for the formation of proteins and other essential biomolecules in living organisms.
Nomenclature of Biomolecules Peptide linkage
The nomenclature of biomolecules with peptide linkage is based on the names of the individual amino acids and the sequence in which they are linked together by peptide bonds.
The individual amino acids are named based on their chemical properties and structure. They are typically named using a three-letter or one-letter code. For example, alanine is abbreviated as Ala or A, and glutamic acid is abbreviated as Glu or E.
The sequence of amino acids in a peptide or protein is typically written from the amino terminus (N-terminus) to the carboxy terminus (C-terminus). The amino acid on the N-terminus has a free amino group, while the amino acid on the C-terminus has a free carboxyl group.
The peptide linkage between two amino acids is represented by a single bond between the carbon atom of the carboxyl group of one amino acid and the nitrogen atom of the amino group of the next amino acid. This bond is often represented using the symbol “–“.
For example, the dipeptide made up of the amino acids alanine and glycine would be named as follows:
N-Ala-Gly-C
This represents the sequence of amino acids from the N-terminus to the C-terminus, with a peptide linkage between the alanine and glycine residues.
Overall, the nomenclature of biomolecules with peptide linkage is based on the names and sequence of the individual amino acids, as well as the specific pattern of peptide linkages between them.
Case Study on Biomolecules Peptide linkage
Case Study: The Role of Peptide Linkage in Insulin Function
Insulin is a hormone produced by the pancreas that regulates glucose metabolism in the body. It is a protein composed of two polypeptide chains, A and B, that are linked together by disulfide bonds and peptide linkages.
The A chain of insulin contains 21 amino acids, while the B chain contains 30 amino acids. The two chains are linked together by two inter-chain disulfide bonds and a single peptide linkage between residues A21 and B1.
This peptide linkage is critical for insulin function because it helps to stabilize the protein and maintain its structure. Without this peptide linkage, the A and B chains would not be properly aligned, and the protein would not be able to bind to its receptor and carry out its biological function.
In addition to the peptide linkage, the disulfide bonds in insulin also play a critical role in its function. These bonds help to maintain the three-dimensional structure of the protein and facilitate its binding to its receptor on target cells.
Insulin function is disrupted in individuals with diabetes, a metabolic disorder characterized by high blood glucose levels. In type 1 diabetes, the body’s immune system attacks and destroys the insulin-producing cells in the pancreas, leading to a deficiency of insulin. In type 2 diabetes, the body becomes resistant to the effects of insulin, and the pancreas is unable to produce enough insulin to compensate.
The discovery of insulin and its role in glucose metabolism revolutionized the treatment of diabetes and has saved countless lives. Understanding the role of peptide linkage and other chemical bonds in insulin function has provided valuable insights into the mechanisms of hormone action and has paved the way for the development of new treatments for diabetes and other metabolic disorders.
White paper on Biomolecules Peptide linkage
Title: Peptide Linkage: The Building Blocks of Life
Introduction:
Biomolecules are the building blocks of life, and peptide linkage is an essential component of many of these molecules. Peptide linkage is the chemical bond that links amino acids together to form peptides and proteins. This white paper will provide an overview of peptide linkage, including its structure, function, and significance in biology.
Structure of Peptide Linkage:
Peptide linkage is formed by a condensation reaction between the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of another amino acid. During this reaction, a molecule of water is released, and a peptide bond is formed between the two amino acids. The resulting molecule is called a dipeptide.
The peptide bond is a planar, covalent bond that links the carbon atom of the carboxyl group to the nitrogen atom of the amino group. The bond has partial double-bond character, which means it is less reactive than a typical single bond. The peptide bond is rigid and planar, which has significant implications for the three-dimensional structure of peptides and proteins.
Function of Peptide Linkage:
Peptide linkage is critical for the formation of peptides and proteins, which are essential biomolecules in living organisms. Peptides and proteins have a wide range of biological functions, including catalysis, signaling, transport, and structural support. The specific sequence of amino acids and the arrangement of peptide linkages determine the properties and functions of these biomolecules.
The sequence of amino acids in a peptide or protein is often referred to as its primary structure. The primary structure determines the secondary and tertiary structure of the protein, which in turn determines its function. The peptide linkage plays a critical role in the secondary and tertiary structure of proteins by determining the folding and stability of the protein.
Significance of Peptide Linkage in Biology:
Peptide linkage is a fundamental concept in biology and has significant implications for our understanding of the structure and function of biomolecules. The discovery of peptide linkage and the chemical bonds that hold proteins together has revolutionized our understanding of the molecular basis of life.
The study of peptide linkage and protein structure has led to many important discoveries, including the development of insulin as a treatment for diabetes, the discovery of enzymes and their catalytic mechanisms, and the identification of signaling pathways that regulate cellular processes.
Conclusion:
Peptide linkage is a critical component of many biomolecules, including peptides and proteins. The peptide bond is a rigid, planar bond that links amino acids together and determines the primary structure of peptides and proteins. Peptide linkage plays a significant role in the structure and function of biomolecules and has revolutionized our understanding of the molecular basis of life. The study of peptide linkage and protein structure continues to be an active area of research with broad implications for biotechnology, medicine, and basic science.