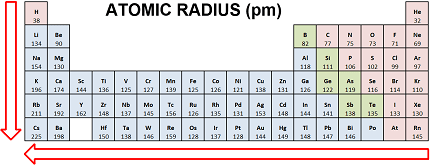
Atomic radius is defined as half the distance between the nuclei of two identical atoms that are bonded together. The size of an atom can be influenced by several factors, including the number of protons in the nucleus, the number of electrons in the atom’s outermost energy level, and the amount of electron shielding provided by inner electrons.
Periodic trends in atomic radius refer to the predictable patterns that emerge as we move across or down the periodic table.
- Across a period (left to right): Atomic radius generally decreases. This is due to the increasing positive charge in the nucleus as the number of protons increases, which attracts the outermost electrons more strongly, pulling them closer to the nucleus and reducing the size of the atom.
- Down a group (top to bottom): Atomic radius generally increases. This is due to the increasing number of energy levels, or shells, as we move down the group. As the number of shells increases, the outermost electrons are farther from the nucleus, reducing the overall attractive force and increasing the size of the atom.
It’s important to note that these trends can be affected by other factors, such as variations in the electron configuration and the presence of transition metals or other unique elements. Nonetheless, they provide a general framework for understanding how atomic radius changes across the periodic table.
What is Required Periodic trends in atomic radius
Required periodic trends in atomic radius refer to the predictable patterns that emerge as we move across or down the periodic table. These trends are important in understanding the behavior of elements and their properties.
The required periodic trends in atomic radius are as follows:
- Across a period (left to right): Atomic radius generally decreases. This is due to the increasing positive charge in the nucleus as the number of protons increases, which attracts the outermost electrons more strongly, pulling them closer to the nucleus and reducing the size of the atom.
- Down a group (top to bottom): Atomic radius generally increases. This is due to the increasing number of energy levels, or shells, as we move down the group. As the number of shells increases, the outermost electrons are farther from the nucleus, reducing the overall attractive force and increasing the size of the atom.
These trends are important because they allow us to predict the behavior and properties of elements based on their position in the periodic table. For example, elements with smaller atomic radii tend to have higher ionization energies and electronegativities, while elements with larger atomic radii tend to have lower ionization energies and electronegativities. Understanding these trends is essential in many fields, including chemistry, materials science, and physics.
When is Required Periodic trends in atomic radius
The concept of Required Periodic trends in atomic radius is always applicable and relevant in the field of chemistry. It is a fundamental concept that is studied in high school and university chemistry courses, as well as in research and industrial applications. The periodic trends in atomic radius are based on the periodic table, which is a fundamental tool used in chemistry to organize and predict the properties of elements based on their atomic structure. These trends can be applied to various chemical reactions and processes, such as the formation of chemical bonds, the reactivity of elements, and the behavior of compounds in different environments. Therefore, the concept of Required Periodic trends in atomic radius is always relevant and important in the study of chemistry.
Where is Required Periodic trends in atomic radius
Required Periodic trends in atomic radius are a fundamental concept in the field of chemistry, and they are applicable to all elements that are listed on the periodic table. The periodic table is a visual representation of the elements, organized in rows and columns based on their atomic structure and properties. The trends in atomic radius are observed when we move across a row (period) or down a column (group) of the periodic table. Therefore, the concept of Required Periodic trends in atomic radius is found in the periodic table and is relevant to the study of chemistry in general. It is taught in chemistry classes, and it is used by chemists and scientists in various applications, such as materials science, pharmaceuticals, and environmental science.
How is Required Periodic trends in atomic radius
Required Periodic trends in atomic radius are the predictable patterns that emerge as we move across or down the periodic table of elements. The atomic radius is the distance between the nucleus of an atom and the outermost electrons. The trends in atomic radius are affected by several factors, including the number of protons in the nucleus, the number of electrons in the outermost energy level, and the amount of electron shielding provided by inner electrons.
- Across a period (left to right): Atomic radius generally decreases. This is because as the number of protons in the nucleus increases, the attractive force on the outermost electrons increases, pulling them closer to the nucleus and reducing the size of the atom.
- Down a group (top to bottom): Atomic radius generally increases. This is because as we move down a group, the number of energy levels increases, and the outermost electrons are farther from the nucleus, reducing the overall attractive force and increasing the size of the atom.
These trends can be observed in the periodic table of elements and are important in understanding the behavior and properties of elements. The trends in atomic radius also impact the reactivity of elements, their electronegativity, and their ability to form chemical bonds. Therefore, understanding Required Periodic trends in atomic radius is crucial in the study of chemistry and its applications.
Nomenclature of Periodic trends in atomic radius
“Nomenclature” refers to the system or set of names used in a particular field or subject. In the case of Required Periodic trends in atomic radius, there is no specific nomenclature or set of names associated with this concept. The terms “atomic radius trends” or “periodic trends in atomic radius” are commonly used to describe the predictable patterns of changes in atomic radius as we move across or down the periodic table.
However, there are some specific terms used to describe the different types of atomic radii that are used in chemistry, such as covalent radius, metallic radius, van der Waals radius, and ionic radius. These terms are used to describe the radius of an atom depending on its specific context, such as in a covalent bond, metallic crystal, or ionic compound.
In general, the nomenclature of Required Periodic trends in atomic radius is relatively straightforward and based on the position of the element in the periodic table. The trend of decreasing atomic radius across a period and increasing atomic radius down a group is a fundamental concept that is widely used and understood in the field of chemistry.
Case Study on Periodic trends in atomic radius
One example of a case study on Required Periodic trends in atomic radius is the analysis of the properties and behavior of elements in Group 2 (also known as the alkaline earth metals) of the periodic table.
Group 2 elements have two valence electrons and are located in the second column from the left on the periodic table. As we move down the group from beryllium (Be) to radium (Ra), the atomic radius increases due to the addition of energy levels. At the same time, the effective nuclear charge (the attractive force experienced by the valence electrons) also increases. These trends can be seen in the following table:
| Element | Atomic radius (pm) |
|---|---|
| Be | 112 |
| Mg | 160 |
| Ca | 197 |
| Sr | 215 |
| Ba | 222 |
| Ra | 223 |
As we move down the group, the increasing atomic radius results in weaker metallic bonding, which leads to lower melting and boiling points. The reactivity of Group 2 elements also increases down the group due to the decrease in ionization energy and the increase in atomic size.
Another important trend is the formation of ionic compounds with Group 2 elements. These elements have a tendency to lose their two valence electrons to form 2+ cations, which then combine with anions to form ionic compounds. The size of the cation and the anion play a significant role in determining the structure and properties of these compounds. For example, smaller cations such as Be2+ tend to form more covalent compounds with smaller anions, while larger cations such as Ba2+ tend to form more ionic compounds with larger anions.
In summary, the study of Required Periodic trends in atomic radius is important in understanding the behavior and properties of elements in the periodic table. The analysis of Group 2 elements provides a specific example of how these trends can be applied to predict and explain the properties and behavior of a group of elements.
White paper on Periodic trends in atomic radius
Title: Understanding Periodic Trends in Atomic Radius: A White Paper
Introduction:
The periodic table of elements is a fundamental tool in the field of chemistry, allowing us to classify and understand the properties and behavior of the elements. One of the key trends observed in the periodic table is the change in atomic radius as we move across or down the table. In this white paper, we will discuss the concept of Required Periodic trends in atomic radius, including the factors that influence these trends, their practical applications, and current research in this area.
Factors Affecting Atomic Radius:
The atomic radius of an element is defined as the distance between the nucleus of the atom and the outermost electrons. The size of the atomic radius is influenced by several factors, including the number of protons in the nucleus, the number of electrons in the outermost energy level, and the amount of electron shielding provided by inner electrons.
Across a period (left to right), the atomic radius generally decreases due to the increased effective nuclear charge experienced by the outermost electrons. Down a group (top to bottom), the atomic radius generally increases due to the addition of energy levels and the increase in electron shielding.
Practical Applications:
The trends in atomic radius have several practical applications in the field of chemistry. For example, these trends can be used to predict the reactivity of elements, their electronegativity, and their ability to form chemical bonds. The trends in atomic radius also impact the properties of metals, such as their ductility, malleability, and electrical conductivity.
One specific example of the practical applications of Required Periodic trends in atomic radius is the analysis of the properties and behavior of elements in Group 2 of the periodic table, as discussed in the case study earlier. Understanding the trends in atomic radius of these elements allows us to predict and explain their properties and behavior in various contexts, such as in ionic compounds or metallic bonding.
Current Research:
There is ongoing research in the field of Required Periodic trends in atomic radius, particularly in the development of new materials with specific properties based on these trends. For example, researchers are exploring the use of certain metals with unique atomic radius characteristics in the development of new superconductors, catalysts, and semiconductors. The precise control of atomic radius and other properties is also important in the development of nanomaterials, which have numerous applications in medicine, electronics, and other fields.
Conclusion:
In conclusion, Required Periodic trends in atomic radius are a fundamental concept in the field of chemistry, allowing us to predict and explain the properties and behavior of elements. These trends are influenced by several factors, including the number of protons, the number of electrons, and the amount of electron shielding. Understanding these trends has practical applications in the analysis and development of materials, and ongoing research is exploring new applications of these trends in various fields.
