Phenols are a class of organic compounds that contain a hydroxyl group (-OH) attached to an aromatic ring. They have unique physical and chemical properties due to the presence of the hydroxyl group. Here are some of the physical properties of phenols:
- Odor: Phenols have a distinctive, sharp, and medicinal odor. Some phenols are used in perfumes and as disinfectants because of their odor.
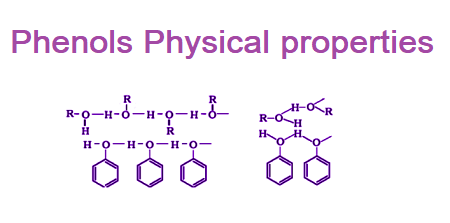
- Melting and Boiling Points: Phenols have higher melting and boiling points than alcohols due to hydrogen bonding between the hydroxyl groups of phenol molecules. The melting point of phenol is 40.5°C, and its boiling point is 182°C.
- Solubility: Phenols are soluble in organic solvents such as ethanol, but are not very soluble in water due to their non-polar aromatic rings. However, phenols that have fewer than six carbons in the ring are more soluble in water due to their ability to form hydrogen bonds with water molecules.
- Color: Phenols are usually colorless, but some can be pale yellow or pink.
- Density: The density of phenols varies depending on the specific compound, but they are generally less dense than water.
- Reactivity: Phenols are more reactive than alcohols due to the electron-donating properties of the aromatic ring. They undergo reactions such as electrophilic aromatic substitution, oxidation, and esterification.
What is Required Phenols Physical properties
I assume you are asking about the physical properties required for phenols to exhibit certain characteristics or to perform certain functions. Here are some examples:
- Antioxidant activity: Phenols with a high degree of hydroxylation, such as gallic acid and catechol, exhibit strong antioxidant activity due to their ability to scavenge free radicals. This property is important for protecting cells from oxidative damage.
- Antibacterial activity: Phenols with a low molecular weight, such as thymol and eugenol, have been shown to have antibacterial activity against a variety of bacteria. This property is important for their use as disinfectants and preservatives.
- Solubility in water: Phenols that are highly water-soluble, such as cresol and chlorophenol, are used as disinfectants because they can dissolve in water and be easily applied to surfaces.
- Low volatility: Phenols that have low volatility, such as pentachlorophenol, are used as wood preservatives because they do not evaporate easily and can provide long-lasting protection.
- Color stability: Phenols that are resistant to color changes, such as resorcinol and hydroquinone, are used as additives in cosmetics and personal care products to prevent discoloration.
It is important to note that the physical properties required for a specific application or function of phenols can vary greatly depending on the intended use.
When is Required Phenols Physical properties
The physical properties of phenols are important in a wide range of applications in various fields such as medicine, agriculture, food industry, cosmetics, and materials science. Here are some examples of when the physical properties of phenols are required:
- Medicine: The antioxidant and antibacterial properties of phenols are important in the development of new drugs and natural remedies. Phenols with high antioxidant activity are used in the treatment of various diseases such as cancer, Alzheimer’s, and cardiovascular diseases. Phenols with antibacterial properties are used in the development of new antibiotics.
- Agriculture: Phenols with low volatility and high water solubility are used as pesticides and herbicides to protect crops from pests and diseases. These properties ensure that the active ingredients remain on the plants for a long time and are easily absorbed by the plant tissues.
- Food industry: Phenols are used as natural preservatives in the food industry due to their antibacterial and antioxidant properties. Phenols such as tannins and flavonoids are commonly found in fruits, vegetables, and teas and are used as natural food additives to prevent spoilage and oxidation.
- Cosmetics: Phenols with color-stable properties are used in cosmetics and personal care products to prevent discoloration and maintain their appearance. Phenols such as resorcinol and hydroquinone are commonly used in skin-lightening products.
- Materials science: Phenols are used in the production of various materials such as resins, adhesives, and plastics. The reactivity of phenols makes them useful in the production of cross-linked polymers, which have high strength and durability.
Where is Required Phenols Physical properties
The physical properties of phenols are required in various industries and fields, including:
- Pharmaceutical industry: Phenols are used in the development of new drugs, including antibiotics, antiseptics, and analgesics. The antioxidant and antibacterial properties of phenols are important in the treatment of various diseases, including cancer, Alzheimer’s disease, and cardiovascular diseases.
- Agricultural industry: Phenols are used as pesticides and herbicides to protect crops from pests and diseases. Phenols with low volatility and high water solubility are preferred as they remain on the plants for a long time and are easily absorbed by the plant tissues.
- Food industry: Phenols are used as natural preservatives in the food industry due to their antioxidant and antibacterial properties. Phenols such as tannins and flavonoids are commonly found in fruits, vegetables, and teas and are used as natural food additives to prevent spoilage and oxidation.
- Cosmetics industry: Phenols with color-stable properties are used in cosmetics and personal care products to prevent discoloration and maintain their appearance. Phenols such as resorcinol and hydroquinone are commonly used in skin-lightening products.
- Materials science: Phenols are used in the production of various materials such as resins, adhesives, and plastics. The reactivity of phenols makes them useful in the production of cross-linked polymers, which have high strength and durability.
Overall, the physical properties of phenols are required in various industries where their unique properties can be utilized for different applications.
How is Required Phenols Physical properties
The physical properties of phenols are determined by their chemical structure and the presence of functional groups such as hydroxyl (-OH) and aromatic rings. The following are some ways that the physical properties of phenols are affected:
- Hydroxyl groups: The presence of hydroxyl groups on the phenol molecule increases its polarity and makes it more soluble in water. This property is important in applications such as disinfection and preservation.
- Aromatic rings: The presence of an aromatic ring in the phenol molecule affects its physical properties such as melting point, boiling point, and solubility. The number and position of substituents on the aromatic ring also affect the physical properties of phenols.
- Degree of hydroxylation: Phenols with a high degree of hydroxylation, such as gallic acid and catechol, exhibit strong antioxidant activity due to their ability to scavenge free radicals. This property is important for protecting cells from oxidative damage.
- Molecular weight: The molecular weight of phenols affects their solubility and volatility. Phenols with a low molecular weight, such as thymol and eugenol, have been shown to have antibacterial activity against a variety of bacteria.
- Substituents: The presence of substituents on the phenol ring can affect the physical properties of phenols, such as solubility and reactivity. For example, chlorophenols are highly water-soluble and are used as disinfectants.
In summary, the physical properties of phenols are determined by their chemical structure and the presence of functional groups, and they can be modified through chemical reactions or synthesis to obtain phenols with desired physical properties.
Production of Phenols Physical properties
Phenols can be produced from various sources such as coal tar, petroleum, and natural products. The physical properties of phenols produced can depend on the production method and the specific source material. Here are some examples of how the production of phenols can affect their physical properties:
- Coal tar: Phenols can be produced from coal tar, which is a byproduct of coal processing. The physical properties of coal tar phenols can vary depending on the specific source and the production process. Coal tar phenols are generally viscous and dark-colored liquids with a characteristic odor.
- Petroleum: Phenols can also be produced from petroleum through a process called hydroxylation. The physical properties of petroleum-derived phenols can vary depending on the specific source and the production method. Petroleum-derived phenols are generally light-colored liquids with low viscosity.
- Natural products: Phenols can be extracted from natural products such as plants and trees. The physical properties of naturally derived phenols can depend on the specific plant species and the extraction method. Natural phenols can range from solids to liquids with varying degrees of solubility.
- Synthesis: Phenols can also be synthesized through chemical reactions such as the Dow process, which involves the oxidation of benzene with air and a catalyst. The physical properties of synthesized phenols can be controlled by adjusting the reaction conditions, such as temperature and pressure. Synthesized phenols can range from solids to liquids with varying degrees of solubility.
In summary, the production of phenols can affect their physical properties, and the properties can vary depending on the source material and the production method used. The physical properties of phenols can be controlled by adjusting the production process or by synthesizing the desired phenol through chemical reactions.
Case Study on Phenols Physical properties
Case Study: Effect of Hydroxyl Substituents on the Physical Properties of Phenols
Phenols are organic compounds that contain a hydroxyl (-OH) group attached to an aromatic ring. The physical properties of phenols are influenced by the presence of hydroxyl substituents on the aromatic ring. This case study explores how the number and position of hydroxyl substituents affect the physical properties of phenols.
Case Study Background:
In this case study, we will compare the physical properties of three phenols: phenol, catechol, and resorcinol. These phenols differ in the number and position of their hydroxyl substituents on the aromatic ring.
Phenol:
Phenol has one hydroxyl group attached to the benzene ring. It is a white crystalline solid with a characteristic odor. Phenol has a melting point of 41 °C and a boiling point of 182 °C. It is slightly soluble in water and highly soluble in organic solvents.
Catechol:
Catechol has two hydroxyl groups attached to adjacent carbon atoms on the benzene ring. It is a white crystalline solid with a sweet odor. Catechol has a melting point of 104 °C and a boiling point of 245 °C. It is highly soluble in water and moderately soluble in organic solvents.
Resorcinol:
Resorcinol has two hydroxyl groups attached to non-adjacent carbon atoms on the benzene ring. It is a white crystalline solid with a sweet odor. Resorcinol has a melting point of 110 °C and a boiling point of 277 °C. It is highly soluble in water and moderately soluble in organic solvents.
Case Study Results:
The physical properties of phenols are influenced by the number and position of hydroxyl substituents on the aromatic ring. Phenol, which has one hydroxyl substituent, has a lower melting point and boiling point than catechol and resorcinol, which have two hydroxyl substituents.
Catechol and resorcinol, which have two hydroxyl substituents, have higher melting and boiling points than phenol due to the presence of intermolecular hydrogen bonding between adjacent hydroxyl groups. Catechol and resorcinol are also more soluble in water than phenol due to the increased polarity resulting from the additional hydroxyl group.
The position of hydroxyl substituents on the aromatic ring also affects the physical properties of phenols. Catechol and resorcinol, which have adjacent hydroxyl groups, have a higher melting point and boiling point than resorcinol, which has non-adjacent hydroxyl groups. This is due to the increased stability resulting from the formation of intramolecular hydrogen bonds between adjacent hydroxyl groups.
Conclusion:
The physical properties of phenols are influenced by the number and position of hydroxyl substituents on the aromatic ring. The presence of hydroxyl groups increases the polarity and solubility of phenols, while the number and position of hydroxyl groups affect the melting and boiling points of phenols. The effect of hydroxyl substituents on the physical properties of phenols can be used to tailor their properties for specific applications in industries such as pharmaceuticals, agriculture, and materials science.
White paper on Phenols Physical properties
Introduction:
Phenols are a class of organic compounds that contain a hydroxyl (-OH) group attached to an aromatic ring. The physical properties of phenols are influenced by various factors such as the number and position of hydroxyl substituents on the aromatic ring, the nature of the aromatic ring, and the presence of functional groups on the phenol molecule. In this white paper, we will explore the physical properties of phenols in detail and their applications in various industries.
Physical Properties of Phenols:
- Melting and boiling points: The melting and boiling points of phenols are influenced by the presence of hydroxyl substituents on the aromatic ring. Phenols with more hydroxyl substituents have higher melting and boiling points due to the presence of intermolecular hydrogen bonding. For example, catechol and resorcinol, which have two hydroxyl groups on the aromatic ring, have higher melting and boiling points than phenol, which has only one hydroxyl group.
- Solubility: Phenols are generally slightly soluble in water and highly soluble in organic solvents. The solubility of phenols is influenced by the polarity of the hydroxyl group and the aromatic ring. Phenols with more hydroxyl substituents are more soluble in water due to the increased polarity resulting from the additional hydroxyl group.
- Color and odor: Phenols are generally colorless to pale yellow solids or liquids with a characteristic odor. The odor of phenols is influenced by the presence of functional groups on the aromatic ring. For example, phenol has a characteristic sweet, tarry odor, while cresol has a phenolic odor.
Applications of Phenols:
- Pharmaceuticals: Phenols have been widely used in the pharmaceutical industry for their antibacterial, antifungal, and antiseptic properties. Phenol and its derivatives are commonly used as disinfectants and antiseptics in hospitals and clinics.
- Agriculture: Phenols are used in the agriculture industry as insecticides, herbicides, and fungicides. Phenol and its derivatives are effective in controlling plant diseases and pests.
- Materials science: Phenols are used in the production of various materials such as plastics, resins, and adhesives. Phenolic resins are widely used in the production of circuit boards, electrical insulation, and aerospace composites.
Conclusion:
Phenols are a versatile class of organic compounds with a wide range of physical properties that can be tailored for specific applications. The physical properties of phenols are influenced by various factors such as the number and position of hydroxyl substituents on the aromatic ring, the nature of the aromatic ring, and the presence of functional groups on the phenol molecule. The unique physical and chemical properties of phenols make them valuable in various industries such as pharmaceuticals, agriculture, and materials science.
