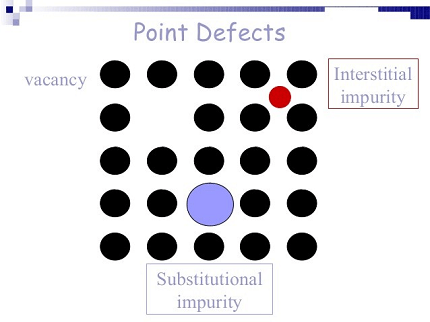
In materials science, point defects refer to localized irregularities in the arrangement of atoms or ions within a crystal lattice structure. These defects can occur naturally during the growth of a crystal, or they can be intentionally introduced through various processes such as irradiation, doping, or mechanical deformation.
There are several types of point defects, including vacancies, interstitials, substitutional impurities, and self-interstitials.
Vacancies are defects that occur when an atom or ion is missing from its expected position within the lattice. These defects can be caused by thermal fluctuations or by the presence of impurities in the crystal.
Interstitials occur when an atom or ion is located at an unexpected site within the lattice, usually in the space between atoms. This type of defect can be introduced by mechanical deformation or radiation damage.
Substitutional impurities are atoms or ions that occupy a lattice site that is normally occupied by a different type of atom or ion. This type of defect can be intentionally introduced by doping a crystal with impurities to alter its properties.
Self-interstitials occur when an atom or ion from the crystal lattice occupies an interstitial site within the same crystal. These defects are typically caused by mechanical deformation or radiation damage.
The presence of point defects can significantly affect the mechanical, electrical, and optical properties of materials. Therefore, understanding and controlling point defects is essential for the development of new materials and the improvement of existing ones.
Vacancy defect
In crystallography, an opening is a sort of point imperfection in a gem where a molecule is absent from one of the cross section locales. Gems innately have flaws, now and then alluded to as crystallographic absconds.
Opening happen normally in every glasslike material. At some random temperature, up to the liquefying point of the material, there is a balance focus (proportion of empty grid locales to those containing molecules). At the softening place of certain metals the proportion can be roughly 1:1000. This temperature reliance can be displayed by
where Nv is the opportunity focus, Qv is the energy expected for opening development, kB is the Boltzmann consistent, T is the outright temperature, and N is the grouping of nuclear destinations for example
where m is mass, NA the Avogadro steady, and M the molar mass.
It is the least difficult point deformity. In this framework, an iota is missing from its customary nuclear site. Opening are shaped during hardening because of vibration of iotas, neighborhood improvement of molecules, plastic disfigurement and ionic bombardments.
The formation of an opening can be basically displayed by considering the energy expected to break the connections between an iota inside the precious stone and its closest neighbor molecules. When that particle is taken out from the grid site, it is placed back on the outer layer of the precious stone and some energy is recovered on the grounds that new bonds are laid out with different iotas on a superficial level. In any case, there is a net contribution of energy since there are less connections between surface particles than between molecules in the inside of the gem.
Frenkel defect
In crystallography, a Frenkel deformity is a kind of point imperfection in glasslike solids, named after its pioneer Yakov Frenkel. The imperfection structures when an iota or more modest particle (typically cation) leaves its position in the cross section, making an opening and turns into an interstitial by housing in a close by area. In basic frameworks, they are essentially produced during molecule illumination, as their development enthalpy is normally a lot higher than for other point surrenders, like opportunities, and hence their harmony focus as per the Boltzmann conveyance is underneath the location limit.[citation needed] In ionic gems, which generally have low dexterity number or a significant divergence in the spans of the particles, this imperfection can be created additionally suddenly, where the more modest particle (typically the cation) is dislocated.[citation needed] Like a Schottky deformity the Frenkel deformity is a stoichiometric imperfection (doesn’t change the over all stoichiometry of the compound). In ionic mixtures, the opening and interstitial imperfection included are oppositely charged and one could anticipate that they should be found near one another because of electrostatic fascination. Be that as it may, this isn’t probable the situation in that frame of mind because of more modest entropy of such a coupled deformity, or on the grounds that the two imperfections could implode into one another. Likewise, on the grounds that such coupled complex imperfections are stoichiometric, their focus will be autonomous of substance conditions.
Interstitial defect
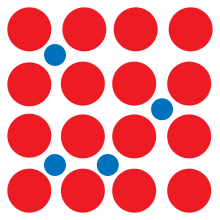
In materials science, an interstitial deformity is a sort of point crystallographic imperfection where a molecule of the equivalent or of an alternate kind, possesses an interstitial site in the precious stone design. At the point when the particle is of similar kind as those generally present they are known as a self-interstitial imperfection. Then again, little iotas in certain precious stones might possess interstitial destinations, like hydrogen in palladium. Interstitials can be delivered by barraging a precious stone with rudimentary particles having energy over the removal limit for that gem, yet they may likewise exist in little focuses in thermodynamic balance. The presence of interstitial imperfections can change the physical and substance properties of a material.
Schottky defect
A Schottky imperfection is an excitation of the site occupations in a gem grid prompting point deserts named after Walter H. Schottky. In ionic gems, this imperfection structures when oppositely charged particles leave their grid locales and become consolidated for example at the surface, making oppositely charged opportunities. These opening are shaped in stoichiometric units, to keep a general nonpartisan charge in the ionic strong.
How is Required Point defects
Point defects are defects in the crystal lattice structure of a material that occur at a specific point in the lattice. Required point defects, also known as intentional point defects or doping, are deliberate additions of impurities or foreign atoms into a material to modify its properties.
There are two main types of required point defects: substitutional and interstitial. Substitutional defects occur when an atom from the lattice is replaced by a foreign atom of similar size, while interstitial defects occur when a foreign atom is inserted into an empty space within the lattice.
The properties of a material can be modified by intentionally introducing specific point defects. For example, doping a semiconductor with impurities can create either p-type or n-type semiconductors, which have different electronic properties and can be used for different applications. Similarly, doping a metal with impurities can change its mechanical properties, such as its strength and ductility.
Overall, required point defects are a useful tool for tailoring the properties of materials for specific applications.
Case Study on Point defects
One example of the importance of point defects in materials science can be seen in the use of doping in semiconductors. Silicon is a common semiconductor material used in electronic devices such as transistors and solar cells. Pure silicon has four valence electrons, which can form covalent bonds with neighboring atoms to create a crystal lattice structure. However, this structure does not conduct electricity very well.
To create a semiconductor that can conduct electricity, silicon can be doped with impurities to intentionally create point defects. For example, adding boron atoms to the silicon lattice can create p-type semiconductors. Boron has one less valence electron than silicon, so when it replaces a silicon atom in the lattice, it creates a “hole” that can conduct positive charge. This makes the material an electron acceptor, creating a p-type semiconductor.
On the other hand, doping silicon with phosphorus atoms can create n-type semiconductors. Phosphorus has one more valence electron than silicon, so when it replaces a silicon atom in the lattice, it creates an extra electron that can conduct negative charge. This makes the material an electron donor, creating an n-type semiconductor.
The controlled introduction of point defects through doping allows for the precise manipulation of the electronic properties of semiconductors. This is essential for the design and fabrication of electronic devices, from computer processors to solar panels.
White paper on Point defects
Introduction
Point defects are structural imperfections that occur within the crystal lattice of materials. They are characterized by the displacement of one or more atoms from their regular lattice sites. Point defects can occur naturally or as a result of external factors such as radiation exposure, temperature changes, or chemical reactions. Point defects are an important area of study in materials science due to their significant impact on the physical and chemical properties of materials. This white paper will provide an overview of point defects, their types, and their effects on material properties.
Types of Point Defects
There are several types of point defects, including vacancies, interstitials, substitutions, and impurities. Vacancies occur when atoms within the lattice are missing from their regular sites. Interstitials are atoms that are located at sites between regular lattice sites. Substitutions occur when an atom is replaced by a different atom of a similar size within the lattice structure. Impurities occur when a foreign atom is introduced into the lattice structure.
Effects of Point Defects
Point defects can have significant effects on the physical and chemical properties of materials. For example, vacancies can increase the diffusion rate of atoms within the lattice, leading to changes in the material’s mechanical and thermal properties. Interstitials can cause the material to become harder, stronger, and more resistant to deformation. Substitutions can change the electronic properties of the material, making it more or less conductive. Impurities can also affect the electronic properties of the material, making it more or less reactive to light, electricity, or other stimuli.
Applications of Point Defects
Point defects are important in a variety of applications in materials science, including semiconductors, superconductors, and radiation-resistant materials. In semiconductors, doping is used to introduce impurities into the lattice structure to create p-type and n-type materials, which are used in the fabrication of electronic devices such as transistors, solar cells, and LEDs. In superconductors, point defects are used to increase the critical temperature at which the material becomes superconductive. In radiation-resistant materials, point defects can be used to prevent or repair damage caused by high-energy radiation.
Conclusion
Point defects are an important area of study in materials science due to their significant impact on the physical and chemical properties of materials. Understanding the types and effects of point defects is essential for the design and development of materials for specific applications. Point defects can be intentionally introduced through doping or impurities to tailor material properties for specific applications. The study of point defects continues to be an active area of research in materials science, with significant potential for future advancements in technology and materials engineering.


