Phenols can be prepared through various methods. Here are some common methods:
- From benzene: Benzene can be oxidized with a mixture of concentrated nitric acid and concentrated sulfuric acid to form nitrobenzene. Nitrobenzene can then be reduced with a reducing agent such as iron and hydrochloric acid to form phenylhydroxylamine. Phenylhydroxylamine can be further oxidized with an oxidizing agent such as chromic acid to form phenol.
- From haloarenes: Haloarenes can be treated with aqueous sodium hydroxide to form phenols via nucleophilic aromatic substitution.
- From cumene: Cumene can be oxidized with air in the presence of a catalyst such as aluminum oxide to form cumene hydroperoxide. Cumene hydroperoxide can then be cleaved with sulfuric acid to form phenol and acetone.
- From diazonium salts: Diazonium salts can be treated with aqueous sodium hydroxide to form phenols via a Sandmeyer reaction.
- From alkylbenzenes: Alkylbenzenes can be treated with aqueous sodium hydroxide in the presence of an oxidizing agent such as potassium permanganate to form phenols.
- From carbohydrates: Carbohydrates such as lignin and tannins can be hydrolyzed with an acid such as sulfuric acid to form phenols.
These are just some of the methods that can be used to prepare phenols. The choice of method will depend on the starting material and the desired product.
What is Required Phenols Preparation
The requirements for preparing phenols can vary depending on the method used. However, some common requirements include:
- Starting material: Depending on the method used, the starting material could be benzene, haloarenes, cumene, diazonium salts, alkylbenzenes, or carbohydrates.
- Reagents: Various reagents are required for different methods. For example, oxidizing agents such as concentrated nitric acid and concentrated sulfuric acid, reducing agents such as iron and hydrochloric acid, and catalysts such as aluminum oxide are required for the oxidation of benzene to phenol. Aqueous sodium hydroxide is required for the nucleophilic aromatic substitution reaction of haloarenes to form phenols. Cumene requires air and a catalyst such as aluminum oxide for oxidation to form cumene hydroperoxide, which can then be cleaved with sulfuric acid to form phenol and acetone.
- Solvents: Depending on the method used, solvents such as water, ether, or benzene may be required.
- Equipment: Depending on the method used, equipment such as reaction vessels, condensers, and reflux apparatus may be required.
- Safety equipment: Some reagents used in phenol preparation can be hazardous, so safety equipment such as gloves, goggles, and lab coats may be required.
Overall, the requirements for phenol preparation will depend on the method used and the starting materials involved.
When is Required Phenols Preparation
Phenols are used in a variety of applications, such as in the production of plastics, pharmaceuticals, and personal care products. Therefore, phenols preparation may be required in industries such as:
- Plastic production: Phenol is used in the production of phenolic resins, which are used to make a variety of plastic products.
- Pharmaceutical production: Phenols are used in the production of various pharmaceuticals such as aspirin and chloramphenicol.
- Personal care product production: Phenols are used in the production of personal care products such as soaps, shampoos, and cosmetics.
- Agrochemical production: Phenols are used in the production of pesticides and herbicides.
- Dye production: Phenols are used in the production of dyes.
- Oil refining: Phenol is used as a catalyst in the refining of petroleum.
- Wood preservation: Phenols are used in the preservation of wood products.
- Adhesive production: Phenols are used in the production of adhesives.
In research, phenols are also used as reagents for various chemical reactions. Therefore, the need for phenol preparation arises in laboratories as well. Overall, the required phenols preparation depends on the specific application and industry involved.
Where is Required Phenols Preparation
Phenols preparation can be carried out in various locations depending on the specific application and industry involved. Some examples include:
- Chemical manufacturing plants: Phenol is produced on an industrial scale in chemical manufacturing plants using various methods such as the cumene process, the Raschig process, and the Dow process.
- Pharmaceutical manufacturing facilities: Phenols are used as starting materials or intermediates in the production of various pharmaceuticals, and their preparation may be carried out in pharmaceutical manufacturing facilities.
- Personal care product manufacturing facilities: Phenols are used in the production of personal care products, and their preparation may be carried out in personal care product manufacturing facilities.
- Research laboratories: Phenols are used as reagents for various chemical reactions in research, and their preparation may be carried out in research laboratories.
- Wood preservation facilities: Phenols are used in the preservation of wood products, and their preparation may be carried out in wood preservation facilities.
- Adhesive manufacturing facilities: Phenols are used in the production of adhesives, and their preparation may be carried out in adhesive manufacturing facilities.
Overall, the location of required phenols preparation depends on the specific application and industry involved.
How is Required Phenols Preparation
Phenols can be prepared through various methods, depending on the starting material and the desired product. Here are some common methods:
- Cumene process: In this method, cumene is oxidized with air in the presence of a catalyst such as aluminum oxide to form cumene hydroperoxide. Cumene hydroperoxide can then be cleaved with sulfuric acid to form phenol and acetone.
- Raschig process: In this method, benzene is sulfonated with sulfuric acid and then treated with sodium hydroxide to form sodium phenoxide. Sodium phenoxide is then acidified to form phenol.
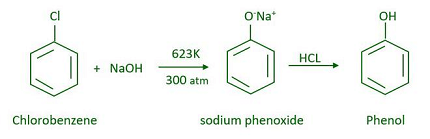
- Dow process: In this method, chlorobenzene is treated with aqueous sodium hydroxide to form sodium phenoxide. Sodium phenoxide is then acidified to form phenol.
- From haloarenes: Haloarenes can be treated with aqueous sodium hydroxide to form phenols via nucleophilic aromatic substitution.
- From benzene: Benzene can be oxidized with a mixture of concentrated nitric acid and concentrated sulfuric acid to form nitrobenzene. Nitrobenzene can then be reduced with a reducing agent such as iron and hydrochloric acid to form phenylhydroxylamine. Phenylhydroxylamine can be further oxidized with an oxidizing agent such as chromic acid to form phenol.
- From carbohydrates: Carbohydrates such as lignin and tannins can be hydrolyzed with an acid such as sulfuric acid to form phenols.
These are just some of the methods that can be used for phenols preparation. The choice of method will depend on the starting material and the desired product. The preparation of phenols may involve various steps such as mixing, heating, cooling, filtration, and purification. The process may be carried out in different types of equipment such as reactors, distillation columns, and extraction vessels. Overall, the specific steps and equipment involved will depend on the chosen method for phenols preparation.
Nomenclature of Phenols Preparation
The nomenclature of phenols preparation typically depends on the method used for their synthesis or the starting material used. Here are some examples:
- Cumene process: The phenol produced via the cumene process is often referred to as cumene hydroperoxide, which is then cleaved to produce phenol and acetone.
- Raschig process: The phenol produced via the Raschig process is often referred to as benzene-1-ol or hydroxybenzene.
- Dow process: The phenol produced via the Dow process is often referred to as chlorobenzene hydroxide or hydroxychlorobenzene.
- From haloarenes: The phenol produced via the reaction of haloarenes with aqueous sodium hydroxide is often referred to as halophenol, where the halogen is indicated by a prefix such as chloro-, bromo-, or iodo-.
- From benzene: The phenol produced via the oxidation of benzene is often referred to as benzene-1,3-diol or 1,3-dihydroxybenzene.
- From carbohydrates: The phenols produced via the hydrolysis of carbohydrates are often referred to as lignin-derived phenols or tannin-derived phenols.
In addition to the above nomenclature, phenols can also be named according to their substituents, which are indicated by prefixes such as methyl-, ethyl-, or propyl-. For example, 4-methylphenol is also known as p-cresol.
Overall, the nomenclature of phenols preparation can vary depending on the method used and the starting material, and may involve a combination of numerical, chemical, and substituent prefixes or suffixes.
Case Study on Phenols Preparation
One example of a case study on phenols preparation is the production of phenol via the cumene process. Phenol is an important chemical used in the production of various products such as resins, adhesives, and plastics. The cumene process is a commonly used industrial method for the production of phenol, and it involves the following steps:
- Cumene oxidation: Cumene (isopropylbenzene) is reacted with air in the presence of a catalyst such as aluminum oxide to produce cumene hydroperoxide.
- Hydroperoxide cleavage: The cumene hydroperoxide is then cleaved with sulfuric acid to produce phenol and acetone.
- Separation and purification: The phenol and acetone mixture is then separated and purified via distillation, extraction, and crystallization to obtain pure phenol.
The cumene process has several advantages, such as high yield, low cost, and high purity of the final product. However, it also has some disadvantages, such as the generation of large amounts of waste and the potential hazards associated with handling and storing cumene hydroperoxide.
One example of a company that produces phenol via the cumene process is INEOS Phenol, which operates a production facility in Germany. The facility has a production capacity of 820,000 tons of phenol per year and uses the cumene process for its production. The company has implemented various measures to minimize the environmental impact of its production process, such as using renewable energy sources and reducing waste generation.
In conclusion, the production of phenol via the cumene process is an important case study in phenols preparation. The process involves several steps, including cumene oxidation, hydroperoxide cleavage, and separation and purification. While the cumene process has several advantages, it also has some disadvantages, and companies such as INEOS Phenol are implementing measures to minimize its environmental impact.
White paper on Phenols Preparation
Introduction:
Phenols are organic compounds that contain a hydroxyl (-OH) group attached to an aromatic ring. Phenols are important industrial chemicals used in the production of a wide range of products such as plastics, resins, adhesives, and pharmaceuticals. There are several methods for the preparation of phenols, each with its own advantages and disadvantages. In this white paper, we will explore some of the most commonly used methods for the preparation of phenols.
Methods for Phenols Preparation:
- Cumene Process: The cumene process is a widely used industrial method for the production of phenol. The process involves the oxidation of cumene (isopropylbenzene) with air to form cumene hydroperoxide, which is then cleaved with sulfuric acid to produce phenol and acetone. The phenol is then separated and purified by distillation, extraction, and crystallization. The cumene process is known for its high yield, low cost, and high purity of the final product. However, it also generates large amounts of waste and requires careful handling and storage of cumene hydroperoxide due to its potential hazards.
- Raschig Process: The Raschig process involves the fusion of sodium phenoxide with benzene sulfonic acid to form phenol and sodium benzene sulfonate. The phenol is then separated and purified by distillation. The Raschig process is less commonly used than the cumene process due to its lower yield and higher cost.
- Dow Process: The Dow process involves the hydrolysis of chlorobenzene to form hydroxychlorobenzene, which is then treated with sodium hydroxide to form phenol and sodium chloride. The phenol is then separated and purified by distillation. The Dow process is known for its high yield and low cost but requires careful handling of chlorobenzene due to its potential hazards.
- From Haloarenes: Phenols can also be prepared by the reaction of haloarenes (halogenated aromatic compounds) with aqueous sodium hydroxide. The haloarene is converted to a phenol via a nucleophilic substitution reaction. The resulting phenol is then separated and purified by distillation or extraction. This method is less commonly used than other methods due to its lower yield and the potential for the formation of unwanted byproducts.
- From Benzene: Phenols can be prepared by the oxidation of benzene with various oxidizing agents such as chromic acid, nitric acid, or ozone. The resulting product is a mixture of phenol and other byproducts, which are separated and purified by distillation or extraction. This method is less commonly used than other methods due to its low yield and high cost.
Conclusion:
The preparation of phenols is an important industrial process with a wide range of applications. There are several methods for the preparation of phenols, each with its own advantages and disadvantages. The cumene process is the most widely used method due to its high yield, low cost, and high purity of the final product. However, companies must be mindful of the potential hazards associated with the handling and storage of cumene hydroperoxide. Other methods, such as the Raschig and Dow processes, are less commonly used due to their lower yield and higher cost. Overall, the choice of method for the preparation of phenols depends on factors such as the desired yield, purity, and cost, as well as the potential hazards associated with the process.
