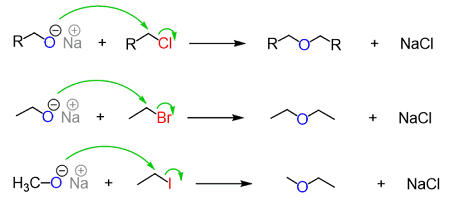
Williamson’s synthesis is a method used to prepare ethers. The general reaction involves the reaction of an alkoxide ion with a primary alkyl halide or a primary alcohol with an alkyl halide in the presence of a strong base such as sodium or potassium hydroxide.
The reaction mechanism involves the formation of an alkoxide ion from the alcohol or anion from the alkoxide in the presence of the strong base. This ion then acts as a nucleophile and attacks the primary alkyl halide to form an intermediate. The intermediate then undergoes elimination of the halide ion to give the ether product.
The reaction can be written as follows:
R-OH + NaOH → R-O⁻Na⁺ + H₂O R-O⁻Na⁺ + RX → R-OR + NaX
where R represents an alkyl group and X represents a halide ion.
This method is a reliable and widely used method for the preparation of symmetrical and unsymmetrical ethers. However, it is important to note that the reaction can be hindered if the alkyl halide is sterically hindered or if the alkoxide ion is not a good nucleophile. In such cases, alternative methods such as the use of acid catalysts or the use of different bases may be required.
What is Required Preparation by Williamson’s synthesis
To perform the Williamson’s synthesis, the following materials are required:
- Primary alkyl halide or primary alcohol: The alkyl halide or alcohol must be primary in nature, as secondary or tertiary alkyl halides are generally unreactive towards alkoxide ions. The choice of alkyl halide or alcohol will depend on the specific ether being synthesized.
- Strong base: A strong base such as sodium or potassium hydroxide is required to generate the alkoxide ion from the alcohol or to activate the alkoxide ion if it is being used as the nucleophile.
- Solvent: The reaction is typically carried out in a polar aprotic solvent such as dimethyl sulfoxide (DMSO), dimethylformamide (DMF), or tetrahydrofuran (THF).
- Apparatus: The reaction can be carried out using simple glassware such as a round-bottom flask, reflux condenser, and magnetic stirrer.
- Workup materials: After the reaction is complete, the ether product must be isolated from the reaction mixture. This is typically done through a workup procedure involving the addition of water, extraction with an organic solvent, and drying over a drying agent such as magnesium sulfate or sodium sulfate.
Overall, the Williamson’s synthesis is a straightforward and reliable method for the preparation of ethers, and can be carried out with relatively simple laboratory equipment and materials.
When is Required Preparation by Williamson’s synthesis
The Williamson’s synthesis is used in organic chemistry for the preparation of ethers. It can be used whenever there is a need to synthesize an ether compound, which could be for a wide range of applications. Some specific examples where Williamson’s synthesis might be used include:
- Synthesis of symmetrical and unsymmetrical ethers for use as solvents, reagents, and intermediates in organic synthesis.
- Preparation of pharmaceutical compounds where ethers are often used as a structural element or functional group.
- Production of fragrance and flavor compounds, where ethers can contribute to the desired aroma or taste.
- Preparation of polymers and materials where ethers can be incorporated as a component to modify the properties of the final product.
Overall, Williamson’s synthesis is a versatile and widely used method for the preparation of ethers, and can be employed in a variety of contexts where the synthesis of ether compounds is required.
Where is Required Preparation by Williamson’s synthesis
The Williamson’s synthesis can be performed in a laboratory setting, typically in a fume hood to provide proper ventilation and safety measures. The reaction can be carried out in a variety of glassware, including round-bottom flasks, reflux condensers, and magnetic stirrers. The reaction requires the use of specific reagents, solvents, and a strong base, and must be performed with appropriate safety measures and personal protective equipment, including gloves, safety glasses, and lab coats. The synthesis can be performed in a variety of settings, including academic research labs, industrial research and development labs, and chemical manufacturing facilities.
How is Required Preparation by Williamson’s synthesis
The Williamson’s synthesis involves the reaction of an alkoxide ion with a primary alkyl halide or a primary alcohol with an alkyl halide in the presence of a strong base such as sodium or potassium hydroxide. The general steps for the synthesis are as follows:
- Formation of the alkoxide ion: The alcohol is deprotonated by the strong base to form the alkoxide ion, which will act as the nucleophile in the reaction.
- Nucleophilic attack: The alkoxide ion attacks the primary alkyl halide or primary alcohol to form an intermediate.
- Elimination: The intermediate undergoes elimination of the halide ion or water to give the ether product.
The reaction can be written as follows:
R-OH + NaOH → R-O⁻Na⁺ + H₂O R-O⁻Na⁺ + RX → R-OR + NaX
where R represents an alkyl group and X represents a halide ion.
The reaction is typically carried out in a polar aprotic solvent such as dimethyl sulfoxide (DMSO), dimethylformamide (DMF), or tetrahydrofuran (THF), and can be heated under reflux to drive the reaction forward.
After the reaction is complete, the ether product must be isolated from the reaction mixture through a workup procedure involving the addition of water, extraction with an organic solvent, and drying over a drying agent such as magnesium sulfate or sodium sulfate.
Overall, the Williamson’s synthesis is a reliable and widely used method for the preparation of ethers, and can be carried out with relatively simple laboratory equipment and materials.
Production of Preparation by Williamson’s synthesis
The Williamson’s synthesis is a well-established method for the production of ethers in both laboratory and industrial settings. The production process typically involves the following steps:
- Selection of reactants: The reactants, which include a primary alkyl halide or primary alcohol, a strong base such as sodium or potassium hydroxide, and a polar aprotic solvent such as dimethyl sulfoxide (DMSO), dimethylformamide (DMF), or tetrahydrofuran (THF), are chosen based on the desired ether product.
- Mixing of reagents: The alkoxide ion is generated from the alcohol or activated if it is being used as the nucleophile. The reaction mixture is typically heated under reflux to drive the reaction forward.
- Workup and purification: After the reaction is complete, the ether product is isolated from the reaction mixture through a workup procedure involving the addition of water, extraction with an organic solvent, and drying over a drying agent such as magnesium sulfate or sodium sulfate. The product is typically purified by distillation or column chromatography.
- Quality control: The purity and identity of the ether product are confirmed by analytical techniques such as gas chromatography (GC), nuclear magnetic resonance (NMR) spectroscopy, and mass spectrometry (MS).
The Williamson’s synthesis is widely used in the production of a variety of ether compounds, including symmetrical and unsymmetrical ethers, which are used as solvents, reagents, and intermediates in organic synthesis, as well as in the production of pharmaceuticals, fragrances, and polymers. The process can be performed on a small scale in a laboratory setting or on a large scale in an industrial setting, depending on the specific application.
Case Study on Preparation by Williamson’s synthesis
Here is a case study on the preparation of an ether using Williamson’s synthesis:
Case study: Preparation of diethyl ether
Diethyl ether is a commonly used organic solvent that is prepared by the Williamson’s synthesis using ethanol and a strong base such as sodium or potassium hydroxide, and ethyl bromide as the alkyl halide. The reaction proceeds as follows:
C2H5OH + NaOH → C2H5O- Na+ + H2O C2H5O- Na+ + C2H5Br → C2H5OC2H5 + NaBr
The reaction is typically carried out in a polar aprotic solvent such as tetrahydrofuran (THF) and heated under reflux to drive the reaction forward. After the reaction is complete, the ether product is isolated from the reaction mixture through a workup procedure involving the addition of water, extraction with an organic solvent, and drying over a drying agent such as magnesium sulfate or sodium sulfate. The product is purified by distillation.
In this case, the preparation of diethyl ether was performed on a small scale in a laboratory setting. Ethanol was mixed with sodium hydroxide in THF, and the mixture was heated under reflux for several hours. Ethyl bromide was then added dropwise to the reaction mixture, and the reaction was allowed to proceed for several more hours. After the reaction was complete, the mixture was cooled and filtered, and the ether product was extracted with diethyl ether and dried over magnesium sulfate. The product was purified by distillation, and its purity and identity were confirmed by analytical techniques such as gas chromatography (GC) and nuclear magnetic resonance (NMR) spectroscopy.
The diethyl ether product was used as a solvent in subsequent reactions, demonstrating the versatility and utility of Williamson’s synthesis in organic chemistry.
White paper on Preparation by Williamson’s synthesis
Here is a white paper on Preparation by Williamson’s synthesis:
Introduction:
Williamson’s synthesis is a widely used method for the preparation of ethers, which are important functional groups in organic chemistry. The synthesis involves the reaction of a primary alkyl halide or primary alcohol with a strong base and a nucleophilic ether to form the corresponding ether product. The reaction is typically performed in a polar aprotic solvent and heated under reflux to drive the reaction forward.
Background:
The Williamson’s synthesis was first described by Alexander Williamson in 1850, and it has since become a popular method for the production of ethers in both laboratory and industrial settings. The reaction proceeds via an SN2 mechanism, in which the strong base deprotonates the alcohol or activates it if it is being used as the nucleophile, and the resulting alkoxide ion attacks the alkyl halide, forming the ether product.
Applications:
Williamson’s synthesis is used in the production of a wide range of ether compounds, including symmetrical and unsymmetrical ethers. Ethers are used as solvents, reagents, and intermediates in organic synthesis, as well as in the production of pharmaceuticals, fragrances, and polymers. For example, diethyl ether is commonly used as a solvent for extractions and as an anesthetic, while dimethyl ether is used as a propellant for aerosols and as a fuel for diesel engines.
Procedure:
The Williamson’s synthesis can be performed on a small scale in a laboratory setting or on a large scale in an industrial setting. The reaction typically involves the mixing of the reactants in a polar aprotic solvent, such as dimethyl sulfoxide (DMSO), dimethylformamide (DMF), or tetrahydrofuran (THF), and heating under reflux. After the reaction is complete, the ether product is isolated from the reaction mixture through a workup procedure involving the addition of water, extraction with an organic solvent, and drying over a drying agent such as magnesium sulfate or sodium sulfate. The product is typically purified by distillation or column chromatography, and its purity and identity are confirmed by analytical techniques such as gas chromatography (GC), nuclear magnetic resonance (NMR) spectroscopy, and mass spectrometry (MS).
Conclusion:
In conclusion, Williamson’s synthesis is a versatile and widely used method for the production of ethers in both laboratory and industrial settings. The reaction is relatively simple and efficient, and it can be used to produce a wide range of ether compounds that are important in various fields of chemistry and industry.