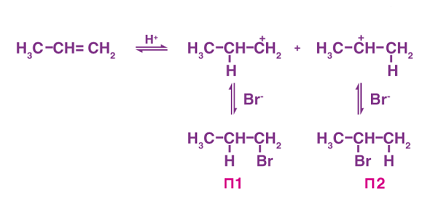
Alkyl halides can be used as starting materials for a wide variety of reactions and transformations. Here are a few examples of preparation methods for alkyl halides:
- Halogenation of alkanes or alkenes: Alkanes and alkenes can be reacted with a halogen (such as chlorine or bromine) to form alkyl halides. This reaction is often carried out in the presence of light or heat to initiate the reaction. For example, ethene (C2H4) can be reacted with hydrogen bromide (HBr) to form ethyl bromide (C2H5Br).
- Alkylation reactions: Alkyl halides can be prepared by reacting an alkene or alkane with a suitable halogenating agent such as phosphorus trichloride (PCl3) or phosphorus pentachloride (PCl5). For example, propane (C3H8) can be reacted with phosphorus trichloride to form 1-chloropropane (C3H7Cl).
- Nucleophilic substitution reactions: Alkyl halides can be prepared by reacting an alcohol or an alkene with a halogen acid (such as hydrochloric acid or hydrobromic acid) or a halogenating agent (such as thionyl chloride or phosphorus tribromide). For example, tert-butyl alcohol (C4H10O) can be reacted with hydrochloric acid (HCl) to form tert-butyl chloride (C4H9Cl).
- Dehydrohalogenation reactions: Alkyl halides can be prepared by reacting an alcohol with a strong acid, such as sulfuric acid (H2SO4), to form an alkyl sulfate intermediate. The alkyl sulfate can then be heated with a strong base, such as potassium hydroxide (KOH), to undergo dehydrohalogenation and form the alkyl halide. For example, tert-butyl alcohol can be reacted with sulfuric acid to form tert-butyl sulfate (C4H9OSO2OH), which can then be heated with potassium hydroxide to form tert-butyl chloride.
These are just a few examples of preparation methods for alkyl halides. The choice of method will depend on the specific alkyl halide needed and the starting material available.
What is Required Preparation from alkyl halides
The required preparation from alkyl halides depends on what reaction or transformation you intend to carry out. Here are a few examples:
- Nucleophilic substitution reactions: If you want to perform a nucleophilic substitution reaction, you may need to prepare the alkyl halide by halogenation of an alkene or alkane. For example, to prepare ethyl bromide (C2H5Br), you can react ethene (C2H4) with hydrogen bromide (HBr) in the presence of a peroxide initiator to form 1-bromoethane (C2H5Br).
- Elimination reactions: If you want to perform an elimination reaction, you may need to prepare the alkyl halide by halogenation of an alcohol. For example, to prepare 1-bromobutane (C4H9Br), you can react 1-butanol (C4H9OH) with hydrobromic acid (HBr) and phosphoric acid (H3PO4) to form the alkyl bromide.
- Grignard reactions: If you want to perform a Grignard reaction, you may need to prepare the alkyl halide by halogenation of an alkane or alkene, or by alkylation of a suitable starting material. For example, to prepare ethylmagnesium bromide (C2H5MgBr), you can react ethyl bromide with magnesium metal in the presence of anhydrous ether.
There are many other reactions and transformations that can be carried out with alkyl halides, each requiring a different preparation method. The choice of preparation method will depend on the specific reaction or transformation you intend to carry out.
When is Required Preparation from alkyl halides
Alkyl halides may be required as starting materials for a wide range of chemical reactions and transformations. Some common examples include:
- Nucleophilic substitution reactions: Alkyl halides are often used as electrophilic substrates in nucleophilic substitution reactions. In these reactions, a nucleophile (such as a hydroxide ion or an amine) displaces the halogen atom from the alkyl halide to form a new bond with the carbon atom. This can be useful for synthesizing a variety of organic compounds, such as alcohols, amines, and carboxylic acids.
- Elimination reactions: Alkyl halides can also be used in elimination reactions, in which a leaving group (such as a halide ion) is removed from the molecule to form a double bond. This can be useful for synthesizing alkenes or alkynes from alkyl halides.
- Grignard reactions: Alkyl halides can be used to prepare organometallic compounds, such as Grignard reagents, which are used as powerful nucleophiles in a variety of reactions. In a Grignard reaction, an alkyl halide reacts with magnesium metal to form an organomagnesium halide, which can then be used to form carbon-carbon bonds with a variety of electrophilic substrates.
- Reductive coupling reactions: Alkyl halides can also be used in reductive coupling reactions, in which two alkyl halides are reacted together in the presence of a reducing agent (such as zinc or magnesium) to form a new carbon-carbon bond. This can be useful for synthesizing complex organic molecules.
Overall, the required preparation of alkyl halides will depend on the specific chemical reaction or transformation being performed.
Where is Required Preparation from alkyl halides
Preparation of alkyl halides can be performed in a variety of settings, including academic research laboratories, industrial production facilities, and pharmaceutical or biotech companies.
In academic research laboratories, preparation of alkyl halides is often carried out as a part of organic synthesis projects. Researchers may need to synthesize specific alkyl halides as intermediates or starting materials for a variety of chemical reactions. These reactions may be used to create new organic compounds, study reaction mechanisms, or investigate biological systems.
In industrial production facilities, alkyl halides may be prepared on a large scale as part of the production process for various chemicals and materials. For example, alkyl halides may be used in the production of plastics, solvents, and pharmaceuticals. The methods used for preparation may be optimized for efficiency and scalability, with a focus on maximizing yield and minimizing costs.
In pharmaceutical and biotech companies, alkyl halides may be used as building blocks in the synthesis of new drugs and drug candidates. These compounds may be designed to interact with specific biological targets or to possess desirable physical and chemical properties for drug development. The preparation of alkyl halides may be an important step in the overall synthesis of these compounds.
How is Required Preparation from alkyl halides
The preparation of alkyl halides typically involves the reaction of an alkyl group with a halogen molecule or a halogen-containing compound. The specific method used for preparation will depend on the starting materials, the desired product, and the reaction conditions.
Here are a few common methods for preparing alkyl halides:
- Halogenation: In this method, an alkane is reacted with a halogen molecule (such as chlorine or bromine) in the presence of heat or light to produce an alkyl halide. The reaction typically proceeds via a free radical mechanism, in which the halogen molecule is split into two halogen radicals that react with the alkane to form the alkyl halide.
- Nucleophilic substitution: In this method, an alkyl halide is synthesized by reacting an alcohol or an alkene with a halogen-containing reagent, such as hydrochloric acid (HCl) or phosphorus tribromide (PBr3). The reaction proceeds via a nucleophilic substitution mechanism, in which the halogen atom is replaced by the nucleophile (such as a chloride ion or a bromide ion) to form the alkyl halide.
- Alkylation: In this method, an alkyl group is reacted with a halogen-containing compound, such as a hydrogen halide or a haloalkane, to form the alkyl halide. The reaction typically proceeds via an S N 2 (substitution nucleophilic bimolecular) mechanism, in which the halide ion acts as a nucleophile and displaces the leaving group (such as a hydroxide ion or a halogen atom) from the alkylating agent.
- Dehydrohalogenation: In this method, an alkyl halide is treated with a strong base (such as potassium hydroxide or sodium methoxide) to remove a hydrogen halide molecule and form an alkene. This method is often used to prepare alkenes from primary or secondary alkyl halides.
Overall, the specific method used for preparing alkyl halides will depend on the starting materials and the desired product, as well as the reaction conditions and any specific constraints or requirements of the synthesis.
Production of Preparation from alkyl halides
The production of alkyl halides typically involves industrial-scale processes that are optimized for efficiency and yield. These processes may use various methods for halogenation, alkylation, and other reactions to produce the desired alkyl halides.
One common method for producing alkyl halides on an industrial scale is through halogenation of alkanes. This process involves reacting the alkane with a halogen gas (such as chlorine or bromine) in the presence of a catalyst and heat or light. The halogenation reaction produces a mixture of different halogenated products, which can then be separated and purified to isolate the desired alkyl halide.
Another method for producing alkyl halides is through alkylation reactions using halogen-containing compounds. For example, a common method for producing alkyl bromides is to react an alkene with hydrogen bromide gas in the presence of a catalyst. This reaction produces the alkyl bromide as the main product, along with some unwanted side products.
In addition to these methods, there are also a variety of other reactions that can be used to produce specific alkyl halides, such as the Sandmeyer reaction, the Finkelstein reaction, and others. These reactions involve various combinations of halogen-containing compounds, catalysts, and other reagents to produce the desired alkyl halide.
Overall, the production of alkyl halides on an industrial scale requires careful optimization of reaction conditions, catalysts, and purification methods to ensure high yields and product purity. These compounds are widely used as intermediates and building blocks in the production of a variety of chemicals, including solvents, plastics, and pharmaceuticals.
Case Study on Preparation from alkyl halides
One notable case study on the preparation of alkyl halides involves the production of ethyl chloride, which is an important intermediate used in the production of a variety of chemicals, including solvents, pharmaceuticals, and insecticides.
Ethyl chloride is typically produced via the reaction of ethylene (C2H4) with hydrogen chloride (HCl) in the presence of a catalyst. This reaction is highly exothermic and typically occurs at temperatures between 400-500 °C, and pressures ranging from 1-4 atmospheres.
The overall reaction can be represented as follows:
C2H4 + HCl → C2H5Cl
The production of ethyl chloride on an industrial scale requires careful optimization of reaction conditions and catalysts to ensure high yields and product purity. For example, the reaction may be carried out in a packed-bed reactor containing a catalyst such as aluminum chloride or iron oxide, which helps to promote the reaction and increase yields.
After the reaction, the resulting mixture of ethyl chloride, unreacted ethylene and hydrogen chloride, and other byproducts is typically separated and purified using distillation or other methods. The ethyl chloride can then be further processed or sold as a chemical intermediate.
Overall, the production of ethyl chloride serves as an important case study in the preparation of alkyl halides, demonstrating the importance of careful optimization of reaction conditions, catalysts, and purification methods to ensure high yields and product purity.
White paper on Preparation from alkyl halides
Introduction:
Alkyl halides, also known as haloalkanes, are organic compounds that contain at least one halogen atom (such as chlorine, bromine, or iodine) attached to a carbon atom in an alkyl group. These compounds are widely used as intermediates and building blocks in the production of a variety of chemicals, including solvents, plastics, and pharmaceuticals.
In this white paper, we will explore the preparation of alkyl halides, with a particular focus on industrial-scale production methods and their applications.
Methods of Preparation:
There are several methods for preparing alkyl halides, including halogenation, alkylation, and other reactions. Halogenation involves reacting an alkane or other hydrocarbon with a halogen gas in the presence of a catalyst and heat or light. The resulting reaction produces a mixture of different halogenated products, which can be separated and purified to isolate the desired alkyl halide.
Alkylation involves reacting a halogen-containing compound with an alkene or other reactive molecule to produce the desired alkyl halide. For example, a common method for producing alkyl bromides is to react an alkene with hydrogen bromide gas in the presence of a catalyst. This reaction produces the alkyl bromide as the main product, along with some unwanted side products.
Other reactions used for the preparation of alkyl halides include the Sandmeyer reaction, which involves the conversion of an aryl or vinyl halide to an alkyl halide using copper salts, and the Finkelstein reaction, which involves the conversion of an alkyl halide from one halogen to another using a salt of the desired halogen.
Industrial Applications:
Alkyl halides have numerous industrial applications, including as solvents, refrigerants, and starting materials for the synthesis of a variety of chemicals. For example, ethyl chloride is an important intermediate used in the production of a variety of chemicals, including solvents, pharmaceuticals, and insecticides. Vinyl chloride is used in the production of polyvinyl chloride (PVC), a widely used plastic.
Another important application of alkyl halides is in the production of agrochemicals, including herbicides, insecticides, and fungicides. For example, chlorpyrifos, an insecticide widely used in agriculture, is produced from the reaction of trichloroethylene with diethylthiophosphoryl chloride.
Conclusion:
In conclusion, the preparation of alkyl halides is an important area of chemical synthesis, with numerous industrial applications. The production of alkyl halides on an industrial scale requires careful optimization of reaction conditions, catalysts, and purification methods to ensure high yields and product purity. As the demand for chemicals continues to grow, the preparation of alkyl halides will continue to play an important role in the chemical industry.
