Hydrogen is a colorless, odorless, and tasteless gas. It is the most abundant element in the universe, constituting about 75% of its elemental mass. Here are some of the properties and uses of hydrogen:
Properties:
- Density: Hydrogen gas is the lightest gas known, and has a density of only 0.0899 grams per liter at standard temperature and pressure.
- Flammability: Hydrogen is highly flammable and burns with a pale blue flame.
- Reactivity: Hydrogen is a highly reactive element and can form compounds with most other elements.
Uses:
- Fuel: Hydrogen gas is used as a fuel in various industrial applications, including in the production of ammonia, methanol, and in fuel cells for electric vehicles.
- Rocket fuel: Hydrogen is also used as a rocket fuel, because it has a high energy density and burns cleanly, producing only water vapor as a byproduct.
- Chemical industry: Hydrogen is used as a reducing agent in the chemical industry, and is also used in the production of chemicals such as hydrochloric acid, ammonia, and methanol.
- Welding: Hydrogen is used as a shielding gas in welding processes, as it can protect the weld area from contamination by oxygen and other atmospheric gases.
- Food industry: Hydrogen is used in the food industry to hydrogenate vegetable oils, which converts them into solid or semi-solid fats for use in margarine, shortening, and other food products.
- Balloons: Hydrogen was once commonly used in balloons, but its use has declined due to its flammability and the risk of explosions.
- Semiconductor industry: Hydrogen is used in the semiconductor industry to clean and etch silicon wafers.
Overall, hydrogen has a wide range of uses in various industries due to its unique properties, such as its high energy density and reactivity.
What is Required Properties and uses of hydrogen
Hydrogen is a chemical element with the symbol H and atomic number 1. It is the lightest and most abundant element in the universe, making up about 75% of its elemental mass. Hydrogen has several unique properties that make it useful in a variety of applications, including:
- High energy density: Hydrogen gas has a high energy content per unit of mass, making it a potentially valuable fuel source for transportation and energy production.
- Non-toxic and non-polluting: When used as a fuel, hydrogen produces only water vapor and heat as byproducts, making it a clean and environmentally friendly energy source.
- Highly flammable: Hydrogen is highly reactive and flammable, which can be both a benefit and a risk in various applications.
- Low boiling and melting points: Hydrogen has a low boiling point of -252.87°C and a low melting point of -259.14°C, which makes it useful in cryogenic applications.
- Ability to form bonds with other elements: Hydrogen is a versatile element that can form bonds with most other elements, allowing it to be used in a wide range of chemical reactions and processes.
In summary, hydrogen has unique properties that make it useful in a variety of applications, including as a fuel source, in cryogenic applications, and in chemical reactions.
.properties
.properties is a document expansion for records fundamentally utilized in Java-related advances to store the configurable boundaries of an application. They can likewise be utilized for putting away strings for Internationalization and confinement; these are known as Property Asset Groups.
Every boundary is put away as a couple of strings, one putting away the name of the boundary (called the key), and the other putting away the worth.
Dissimilar to numerous well known record designs, there is no RFC for .properties documents and detail archives are not generally clear, in all likelihood because of the straightforwardness of the configuration.
List of materials properties
A materials property is an escalated property of a material, i.e., an actual property that doesn’t rely upon how much the material. These quantitative properties might be utilized as a measurement by which the advantages of one material versus another can measure up, in this way supporting materials determination.
A property might be a steady or might be an element of at least one free factors, like temperature. Materials properties frequently change somewhat as per the course in the material wherein they are estimated, a condition alluded to as anisotropy. Materials properties that connect with various actual peculiarities frequently act directly (or roughly so) in a given working range[further clarification needed]. Demonstrating them as direct capabilities can fundamentally work on the differential constitutive conditions that are utilized to depict the property.
Conditions depicting important materials properties are frequently used to foresee the characteristics of a framework.
The properties are estimated by government sanctioned test strategies. Numerous such techniques have been archived by their particular client networks and distributed through the Web; see ASTM Worldwide.
Properties of water
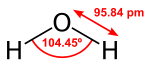

Water (H2O) is a polar inorganic compound that is at room temperature a dull and unscented fluid, which is almost boring separated from an intrinsic touch of blue. It is by a wide margin the most concentrated on substance compound and is depicted as the “general dissolvable” and the “dissolvable of life”. It is the most bountiful substance on the outer layer of Earth and the main normal substance to exist as a strong, fluid, and gas on Earth’s surface. It is additionally the third most plentiful atom known to mankind (behind sub-atomic hydrogen and carbon monoxide).
Water particles structure hydrogen bonds with one another and are unequivocally polar. This extremity permits it to separate particles in salts and cling to other polar substances, for example, alcohols and acids, subsequently dissolving them. Its hydrogen holding causes its numerous special properties, for example, having a strong structure less thick than its fluid structure, a moderately high limit of 100 °C for its molar mass, and a high intensity limit.
Water is amphoteric, implying that it can show properties of a corrosive or a base, contingent upon the pH of the arrangement that it is in; it promptly delivers both H+ and OH− particles. Connected with its amphoteric person, it goes through self-ionization. The result of the exercises, or roughly, the centralizations of H+ and OH− is a consistent, so their particular focuses are contrarily corresponding to one another.
Physical properties
Water is the compound substance with synthetic equation H
2O; one particle of water has two hydrogen molecules covalently clung to a solitary oxygen iota. Water is a boring, unscented fluid at surrounding temperature and tension. Fluid water has frail retention groups at frequencies of around 750 nm which make it seem to have a blue tone. This can undoubtedly be seen in a water-filled shower or wash-bowl whose coating is white. Huge ice precious stones, as in ice sheets, likewise seem blue.
Under standard circumstances, water is basically a fluid, dissimilar to other similar to hydrides of the oxygen family, which are for the most part vaporous. This extraordinary property of water is because of hydrogen holding. The particles of water are continually moving concerning one another, and the hydrogen bonds are persistently breaking and improving at timescales quicker than 200 femtoseconds (2 × 10−13 seconds). Nonetheless, these bonds are sufficiently able to make a significant number of the curious properties of water, some of which make it fundamental to life.
Case Study on Properties and uses of hydrogen
One notable application of hydrogen is in the development of fuel cell technology. Fuel cells use a chemical reaction between hydrogen and oxygen to produce electricity, with water as the only byproduct. This process is highly efficient and produces zero emissions, making fuel cells a promising clean energy technology for transportation and stationary power generation.
However, the properties of hydrogen present some challenges for its use in fuel cells. Hydrogen has a low energy density by volume, which means that it needs to be stored at high pressures or in liquid form to provide enough energy for practical use. Additionally, hydrogen gas is highly flammable and can pose safety risks if not handled properly.
To address these challenges, researchers and companies are developing innovative solutions to improve the properties and uses of hydrogen. For example, materials scientists are developing new materials to safely store hydrogen at lower pressures and improve its energy density. Engineers are also working on designing more efficient fuel cell systems and developing technologies to produce hydrogen from renewable sources such as water and biomass.
In summary, the properties and uses of hydrogen offer both benefits and challenges for its application in fuel cell technology and other industries. Ongoing research and development efforts are focused on improving the safety, efficiency, and environmental sustainability of hydrogen-based technologies.
White paper on Properties and uses of hydrogen
Certainly, I can provide you with an overview of the properties and uses of hydrogen in the form of a white paper.
Introduction:
Hydrogen is a chemical element with the symbol H and atomic number 1. It is the lightest and most abundant element in the universe and has a variety of unique properties that make it useful in many applications. In this white paper, we will explore the properties and uses of hydrogen, including its physical and chemical properties, its use as a fuel, and its potential as a clean energy source.
Properties of Hydrogen:
Hydrogen gas has several unique properties that make it useful in various applications. These include:
- High energy density: Hydrogen gas has a high energy content per unit of mass, making it a valuable fuel source for transportation and energy production.
- Non-toxic and non-polluting: When used as a fuel, hydrogen produces only water vapor and heat as byproducts, making it a clean and environmentally friendly energy source.
- Highly flammable: Hydrogen is highly reactive and flammable, which can be both a benefit and a risk in various applications.
- Low boiling and melting points: Hydrogen has a low boiling point of -252.87°C and a low melting point of -259.14°C, which makes it useful in cryogenic applications.
- Ability to form bonds with other elements: Hydrogen is a versatile element that can form bonds with most other elements, allowing it to be used in a wide range of chemical reactions and processes.
Uses of Hydrogen:
Hydrogen has several important applications in various industries. Some of the most notable uses of hydrogen include:
- Fuel for transportation: Hydrogen can be used as a fuel for fuel cell vehicles, which use a chemical reaction between hydrogen and oxygen to produce electricity. This process is highly efficient and produces zero emissions, making fuel cell vehicles a promising clean energy technology.
- Energy production: Hydrogen can also be used as a fuel for power generation in fuel cells or in combustion engines. It can also be used to store and transport energy from renewable sources such as wind and solar.
- Chemical production: Hydrogen is used in the production of a wide range of chemicals, including ammonia, methanol, and hydrogen peroxide.
- Metals processing: Hydrogen is used in the production of metals such as steel and titanium.
- Cryogenics: Hydrogen’s low boiling and melting points make it useful in cryogenic applications, such as in cooling materials to very low temperatures.
Conclusion:
In summary, the properties and uses of hydrogen make it a versatile and valuable element in various industries. Its high energy density, non-toxic and non-polluting nature, and ability to form bonds with other elements make it a promising candidate for clean energy production and transportation. Ongoing research and development efforts are focused on improving the safety, efficiency, and environmental sustainability of hydrogen-based technologies, which could have a significant impact on the future of energy and industry.
