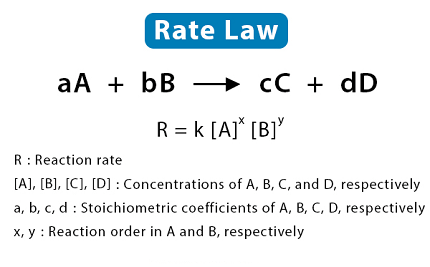
A rate law is an equation that describes how the rate of a chemical reaction depends on the concentrations of the reactants. It is also known as the rate equation or the rate expression.
The general form of a rate law is:
Rate = k [A]^m [B]^n
Where:
- Rate is the rate of the reaction
- k is the rate constant, which depends on the temperature, solvent, and other factors
- [A] and [B] are the concentrations of the reactants A and B, respectively
- m and n are the orders of the reaction with respect to A and B, respectively.
The orders m and n are usually determined experimentally by measuring the rate of the reaction at different concentrations of the reactants and then analyzing the data using various methods such as the method of initial rates or the method of integrated rate laws.
Overall, rate laws are essential tools for understanding the kinetics of chemical reactions and for designing chemical processes in various fields such as chemical engineering, pharmaceuticals, and materials science.
What is Required Rate law
A required rate law refers to the specific form of the rate law equation that is needed to match experimental data for a given chemical reaction.
In order to determine the required rate law, experimental data is collected by measuring the rate of the reaction at different concentrations of reactants. These measurements are used to determine the orders of the reaction with respect to each reactant, as well as the value of the rate constant, k.
Once the orders and rate constant have been determined, the required rate law can be written in the form:
Rate = k [A]^m [B]^n
where [A] and [B] are the concentrations of reactants A and B, respectively, and m and n are the orders of the reaction with respect to A and B, respectively.
The required rate law is important because it provides a quantitative understanding of how the rate of the reaction depends on the concentrations of the reactants, which is crucial for designing chemical processes and predicting reaction outcomes in various fields of chemistry, such as chemical engineering, pharmaceuticals, and materials science.
When is Required Rate law
The required rate law is used in the field of chemistry when determining the specific form of the rate law equation that is needed to match experimental data for a given chemical reaction. This is done by measuring the rate of the reaction at different concentrations of reactants and determining the orders of the reaction with respect to each reactant, as well as the rate constant, k. Once these parameters have been determined, the required rate law can be written in the form:
Rate = k [A]^m [B]^n
This equation describes how the rate of the reaction depends on the concentrations of the reactants A and B, and the orders m and n, respectively. The required rate law is important in understanding the kinetics of chemical reactions and designing chemical processes in various fields such as chemical engineering, pharmaceuticals, and materials science.
Where is Required Rate law
The required rate law is used in the field of chemistry to describe the specific form of the rate law equation that is needed to match experimental data for a given chemical reaction. It is used in various settings such as in academic research, in industry for the development of new chemical products and processes, and in regulatory agencies for the evaluation of the safety and efficacy of drugs and other chemicals. The required rate law can be determined in a laboratory setting by measuring the rate of the reaction at different concentrations of reactants and analyzing the data to determine the orders of the reaction with respect to each reactant and the rate constant. Once the required rate law has been determined, it can be used to predict the behavior of the reaction under different conditions and to design chemical processes that are efficient and safe.
How is Required Rate law
The required rate law is determined experimentally by measuring the rate of the reaction at different concentrations of reactants and analyzing the data to determine the orders of the reaction with respect to each reactant and the rate constant. The following steps are generally involved in determining the required rate law:
- Conduct a series of experiments: The rate of the reaction is measured at different concentrations of the reactants by taking a series of measurements at different time intervals.
- Determine the orders of the reaction: The orders of the reaction with respect to each reactant are determined by analyzing the rate data using methods such as the method of initial rates or the method of integrated rate laws.
- Determine the rate constant: The rate constant, k, is determined by using the values obtained in step 2 and substituting them into the rate law equation.
- Write the required rate law: Once the orders and rate constant have been determined, the required rate law can be written in the form:
Rate = k [A]^m [B]^n
where [A] and [B] are the concentrations of reactants A and B, respectively, and m and n are the orders of the reaction with respect to A and B, respectively.
In summary, the required rate law is determined through experimentation and data analysis to provide a quantitative understanding of how the rate of a chemical reaction depends on the concentrations of the reactants, which is crucial for designing chemical processes and predicting reaction outcomes in various fields of chemistry.
Nomenclature of Rate law
The nomenclature of the rate law describes the notation used to represent the rate law equation for a chemical reaction. The rate law equation represents the mathematical relationship between the rate of the reaction and the concentrations of the reactants. The general form of the rate law equation is:
Rate = k [A]^m [B]^n
Where:
- Rate: is the rate of the reaction
- k: is the rate constant or specific rate constant
- [A] and [B]: are the concentrations of the reactants A and B, respectively
- m and n: are the reaction orders with respect to A and B, respectively.
The nomenclature of the rate law includes the symbols and terminology used to represent each of these variables. For example, Rate is commonly represented using the symbol “r” or “v”, k is usually represented using the symbol “k”, and the concentrations of reactants are represented using brackets around the chemical symbol, such as [A] and [B]. The reaction orders are represented using the symbols “m” and “n” and are typically determined experimentally.
In summary, the nomenclature of the rate law includes the symbols and terminology used to represent the variables in the rate law equation, which is crucial for understanding the mathematical relationship between the rate of the reaction and the concentrations of the reactants.
Case Study on Rate law
Here is a case study on the determination of the rate law for a chemical reaction:
Case Study: Determination of the Rate Law for the Reaction between Iodine and Propanone
The reaction between iodine and propanone in acidic solution is a well-known example of a chemical reaction whose rate can be expressed using a rate law equation. The reaction is given by the following equation:
I2(aq) + CH3COCH3(aq) + H+(aq) → CH3COCH2I(aq) + HI(aq)
The rate law for this reaction can be determined experimentally by measuring the initial rate of the reaction under different conditions and analyzing the data to determine the orders of the reaction with respect to each reactant and the rate constant. Here are the steps involved in determining the rate law for this reaction:
- Preparation of the reaction mixture: A solution of iodine, propanone, and sulfuric acid is prepared and allowed to equilibrate to a constant temperature.
- Determination of the initial rate: The initial rate of the reaction is determined by measuring the amount of iodine that reacts with propanone over a fixed period of time using a spectrophotometer. This measurement is repeated for different initial concentrations of iodine, propanone, and sulfuric acid.
- Analysis of the data: The rate data is analyzed using the method of initial rates to determine the order of the reaction with respect to iodine and propanone. The method of initial rates involves measuring the initial rate of the reaction at different initial concentrations of each reactant while keeping the concentration of the other reactant constant. The order of the reaction with respect to each reactant is determined by comparing the initial rates obtained under these conditions.
- Determination of the rate constant: The rate constant, k, is determined by using the values obtained in step 3 and substituting them into the rate law equation.
- Writing the rate law equation: Once the orders and rate constant have been determined, the rate law equation can be written as follows:
Rate = k[I2]^x [CH3COCH3]^y [H+]^z
where [I2], [CH3COCH3], and [H+] are the concentrations of iodine, propanone, and hydrogen ions, respectively, and x, y, and z are the orders of the reaction with respect to each reactant, which were determined in step 3.
In summary, the rate law for the reaction between iodine and propanone was determined experimentally by measuring the initial rate of the reaction under different conditions and analyzing the data to determine the orders of the reaction with respect to each reactant and the rate constant. This case study highlights the importance of understanding the kinetics of chemical reactions and the role of rate laws in describing their behavior.
White paper on Rate law
Here is a white paper on the topic of Rate Laws:
Introduction:
Rate laws are a fundamental part of chemical kinetics, which is the study of the rates and mechanisms of chemical reactions. A rate law describes the mathematical relationship between the rate of a chemical reaction and the concentrations of the reactants. The rate law equation can be used to predict the rate of a reaction under different conditions and to understand the factors that influence the rate of a reaction. In this white paper, we will discuss the concept of rate laws, including their importance, factors that affect reaction rates, and the experimental determination of rate laws.
Importance of Rate Laws:
Rate laws are important because they provide a quantitative description of the rates of chemical reactions. The rate of a reaction is the speed at which reactants are converted into products, and it is a crucial parameter in many areas of chemistry, including chemical synthesis, materials science, and environmental chemistry. Rate laws allow us to predict the rates of chemical reactions under different conditions, which is essential for designing chemical processes and understanding the behavior of chemical systems.
Factors Affecting Reaction Rates:
Several factors can affect the rate of a chemical reaction, including temperature, concentration, surface area, and the presence of a catalyst. Temperature is one of the most important factors that affect reaction rates. Increasing the temperature usually increases the rate of a reaction because it increases the kinetic energy of the reactant molecules, which makes them more likely to collide and react. Concentration is another important factor that affects reaction rates. Increasing the concentration of the reactants usually increases the rate of a reaction because it increases the number of collisions between reactant molecules. Surface area is also important because it increases the number of reactive sites available for reaction. Finally, catalysts can increase the rate of a reaction by lowering the activation energy required for the reaction to occur.
Experimental Determination of Rate Laws:
The experimental determination of rate laws involves measuring the initial rates of a reaction under different conditions and analyzing the data to determine the orders of the reaction with respect to each reactant and the rate constant. The orders of the reaction with respect to each reactant can be determined using the method of initial rates or the method of integrated rate laws. The method of initial rates involves measuring the initial rate of the reaction at different initial concentrations of each reactant while keeping the concentration of the other reactant constant. The order of the reaction with respect to each reactant is determined by comparing the initial rates obtained under these conditions. The method of integrated rate laws involves measuring the concentrations of reactants or products at different times during the reaction and analyzing the data to determine the order of the reaction with respect to each reactant and the rate constant.
Conclusion:
In conclusion, rate laws are an essential part of chemical kinetics, which is the study of the rates and mechanisms of chemical reactions. Rate laws provide a quantitative description of the rates of chemical reactions and allow us to predict the rates of reactions under different conditions. Several factors can affect reaction rates, including temperature, concentration, surface area, and the presence of a catalyst. Finally, the experimental determination of rate laws involves measuring the initial rates of a reaction under different conditions and analyzing the data to determine the orders of the reaction with respect to each reactant and the rate constant.
