The reaction with nitrous acid (HNO2) depends on the conditions and the nature of the compound with which it reacts. In general, nitrous acid is a weak acid and a good oxidizing agent. Here are a few examples of reactions with nitrous acid:
- Reaction with alcohols: Nitrous acid reacts with alcohols to produce alkyl nitrites. For example, ethyl alcohol reacts with nitrous acid to form ethyl nitrite:
CH3CH2OH + HNO2 → CH3CH2ONO
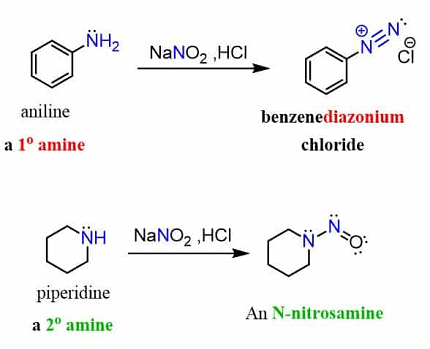
- Reaction with primary amines: Primary amines react with nitrous acid to form diazonium salts. For example, aniline reacts with nitrous acid to form diazonium chloride:
C6H5NH2 + HNO2 → C6H5N2Cl + 2H2O
- Reaction with secondary amines: Secondary amines react with nitrous acid to form nitrosamines. For example, dimethylamine reacts with nitrous acid to form N-nitrosodimethylamine:
(CH3)2NH + HNO2 → (CH3)2NNO + H2O
- Reaction with phenols: Phenols react with nitrous acid to form nitroso compounds. For example, phenol reacts with nitrous acid to form 2-nitrosophenol:
C6H5OH + HNO2 → C6H5NO + H2O
In summary, the reaction with nitrous acid can result in the formation of various products depending on the reactants and conditions.
What is Required Amines Reaction with nitrous acid
The reaction between nitrous acid (HNO2) and amines results in the formation of diazonium salts. However, not all amines can undergo this reaction. Only primary aromatic amines and aliphatic amines can undergo this reaction to form diazonium salts. Secondary amines and tertiary amines do not react with nitrous acid to form diazonium salts.
The reaction between nitrous acid and a primary amine involves the following steps:
- The nitrous acid is protonated to form the nitrosonium ion (NO+), which is the electrophile in the reaction:
HNO2 + H+ → NO+ + H2O
- The primary amine reacts with the nitrosonium ion to form the diazonium salt:
R-NH2 + NO+ → R-N2+ + H2O
where R is an aryl or alkyl group.
For example, aniline (C6H5NH2) reacts with nitrous acid to form the diazonium salt of aniline, which is diazonium chloride:
C6H5NH2 + HNO2 → C6H5N2Cl + 2H2O
Overall, the reaction of nitrous acid with primary aromatic or aliphatic amines is a useful synthetic method for the preparation of diazonium salts, which can be used in various organic reactions.
When is Required Amines Reaction with nitrous acid
The reaction between amines and nitrous acid is used for the preparation of diazonium salts, which are versatile intermediates in various organic reactions. The diazonium salts are used to introduce different functional groups, such as halogens, hydroxyl groups, amino groups, and cyano groups, onto the aromatic ring. The reaction is specific to primary aromatic and aliphatic amines.
The reaction between nitrous acid and primary aromatic amines is commonly used in the synthesis of azo dyes, which are extensively used in the textile industry. Azo dyes are characterized by the presence of a diazo group (-N=N-) in the molecule, which is introduced by the reaction of a primary aromatic amine with nitrous acid.
The reaction between nitrous acid and primary aliphatic amines is less commonly used than the reaction with primary aromatic amines. The diazonium salts formed from primary aliphatic amines are less stable than those formed from primary aromatic amines and are more prone to decomposition.
In summary, the reaction between amines and nitrous acid is a useful synthetic method for the preparation of diazonium salts, which can be used in various organic reactions. The reaction is specific to primary aromatic and aliphatic amines and is commonly used in the synthesis of azo dyes.
Where is Required Amines Reaction with nitrous acid
The reaction between amines and nitrous acid is a well-known organic reaction and can be carried out in a laboratory setting. The reaction typically requires the use of nitrous acid, which can be generated in situ by adding sodium nitrite (NaNO2) to an acidic solution. The acidic solution is usually prepared by adding hydrochloric acid (HCl) or sulfuric acid (H2SO4) to water. The amine is then added to the solution, and the mixture is stirred at a low temperature (0-5°C) to prevent the decomposition of the diazonium salt.
The reaction between nitrous acid and primary aromatic amines is typically carried out in an ice bath, as the reaction is exothermic and can lead to the formation of by-products if the temperature is not controlled. The reaction between nitrous acid and primary aliphatic amines is less exothermic and can be carried out at room temperature.
In summary, the reaction between amines and nitrous acid can be carried out in a laboratory setting using nitrous acid generated in situ by adding sodium nitrite to an acidic solution. The reaction is typically carried out at low temperatures to prevent decomposition of the diazonium salt.
How is Required Amines Reaction with nitrous acid
The reaction between amines and nitrous acid involves the formation of diazonium salts, which are useful intermediates in organic synthesis. The reaction is specific to primary aromatic and aliphatic amines and proceeds through the following steps:
- Protonation of nitrous acid: The nitrous acid (HNO2) is protonated in an acidic solution to form the nitrosonium ion (NO+), which is the electrophile in the reaction. The acid used is typically hydrochloric acid (HCl) or sulfuric acid (H2SO4), and the reaction is usually carried out at low temperatures to prevent decomposition of the diazonium salt.
HNO2 + H+ → NO+ + H2O
- Formation of the diazonium salt: The primary amine is then added to the solution, and the nitrosonium ion reacts with the amine to form the diazonium salt. The reaction is specific to primary aromatic and aliphatic amines.
R-NH2 + NO+ → R-N2+ + H2O
where R is an aryl or alkyl group.
For example, the reaction of aniline with nitrous acid generates the diazonium salt of aniline, which is diazonium chloride:
C6H5NH2 + HNO2 → C6H5N2Cl + 2H2O
The reaction between nitrous acid and primary aliphatic amines is less commonly used and leads to less stable diazonium salts, which are prone to decomposition.
Overall, the reaction between amines and nitrous acid is a useful synthetic method for the preparation of diazonium salts, which can be used in various organic reactions.
Production of Amines Reaction with nitrous acid
The reaction between nitrous acid and amines can be used to produce primary aromatic amines from nitro compounds. This is known as the “reduction of nitro compounds with nitrous acid” or “Zincke reaction”. The reaction involves the following steps:
- Formation of nitrosonium ion: Nitrous acid is protonated in an acidic solution to form the nitrosonium ion (NO+), which is the electrophile in the reaction.
HNO2 + H+ → NO+ + H2O
- Formation of diazonium salt: The nitro compound is added to the solution, and the nitrosonium ion reacts with the nitro compound to form a diazonium salt.
Ar-NO2 + NO+ → Ar-N2+ + H2O
where Ar is an aromatic group.
- Reduction of diazonium salt: The diazonium salt is then reduced to form the primary aromatic amine using a reducing agent, such as hydrochloric acid and zinc dust.
Ar-N2+ + H2O + Zn → Ar-NH2 + Zn(OH)Cl
Overall, the Zincke reaction is a useful method for the production of primary aromatic amines from nitro compounds using nitrous acid as a key reagent.
Case Study on Amines Reaction with nitrous acid
One interesting case study involving the reaction between amines and nitrous acid is the synthesis of azo dyes. Azo dyes are a class of organic compounds that contain a characteristic azo (-N=N-) functional group, which is formed by the reaction of two aromatic amines with nitrous acid. The reaction is commonly known as diazotization and coupling.
In the first step of the diazotization reaction, the amine is treated with nitrous acid to form the corresponding diazonium salt:
Ar-NH2 + HNO2 → Ar-N2+ + 2H2O
where Ar is an aromatic group.
In the second step of the reaction, the diazonium salt is coupled with a second aromatic amine or aniline derivative to form the azo compound:
Ar-N2+ + Ar’-NH2 → Ar-N=N-Ar’ + 2H+
where Ar’ is a different aromatic group.
The reaction can be used to produce a wide range of azo dyes with various colors, depending on the choice of the aromatic amines used. For example, the coupling of diazonium salt of 2-naphthol with aniline produces an orange azo dye called Sudan I, which is commonly used as a food dye. Similarly, coupling of diazonium salt of 4-aminobenzenesulfonic acid with 2-naphthol produces a red azo dye called Ponceau 4R.
The diazotization and coupling reaction is an important method for the synthesis of azo dyes, which are widely used in the textile, food, and cosmetic industries. However, the reaction must be carefully controlled to prevent the formation of undesired by-products or decomposition of the diazonium salt. Additionally, the use of nitrous acid in large-scale industrial processes raises concerns regarding the safety and environmental impact of the reaction.
White paper on Amines Reaction with nitrous acid
Introduction:
The reaction between amines and nitrous acid is a useful tool in organic chemistry for the preparation of diazonium salts, which can be used as intermediates in various organic reactions. The reaction is specific to primary aromatic and aliphatic amines and involves the formation of diazonium salts through the protonation of nitrous acid, followed by the addition of a primary amine. In this white paper, we will discuss the mechanism, applications, and limitations of the amines reaction with nitrous acid.
Mechanism:
The reaction between nitrous acid and a primary amine proceeds through a mechanism involving the protonation of nitrous acid and the formation of a diazonium salt. The mechanism can be described in the following steps:
- Protonation of nitrous acid: Nitrous acid is protonated in an acidic solution to form the nitrosonium ion (NO+), which is the electrophile in the reaction.
HNO2 + H+ → NO+ + H2O
- Formation of diazonium salt: The primary amine is then added to the solution, and the nitrosonium ion reacts with the amine to form the diazonium salt.
R-NH2 + NO+ → R-N2+ + H2O
where R is an aryl or alkyl group.
Applications:
The reaction between amines and nitrous acid is a useful method for the preparation of diazonium salts, which are important intermediates in organic synthesis. Diazonium salts can be used in various organic reactions, such as:
- Coupling reactions: Diazonium salts can be coupled with other aromatic compounds, such as phenols, to form azo compounds, which are widely used as dyes in the textile industry.
- Substitution reactions: Diazonium salts can be used as electrophiles in substitution reactions with nucleophiles such as halides, cyanides, and carboxylates.
- Reduction reactions: Diazonium salts can be reduced to form arylamines, which are useful intermediates in the synthesis of pharmaceuticals and agrochemicals.
Limitations:
The reaction between amines and nitrous acid is specific to primary aromatic and aliphatic amines and is not applicable to secondary or tertiary amines. Additionally, the reaction can lead to the formation of unstable diazonium salts, which are prone to decomposition. Careful control of the reaction conditions, such as temperature and pH, is required to prevent the formation of unwanted by-products and to ensure the stability of the diazonium salt.
Conclusion:
The reaction between amines and nitrous acid is a useful tool in organic synthesis for the preparation of diazonium salts, which are versatile intermediates in various organic reactions. The reaction involves the protonation of nitrous acid, followed by the addition of a primary amine to form the diazonium salt. The reaction has several applications in organic synthesis, but care must be taken to prevent the formation of unwanted by-products and to ensure the stability of the diazonium salt. Overall, the amines reaction with nitrous acid is a valuable tool in the synthetic chemist’s toolbox.