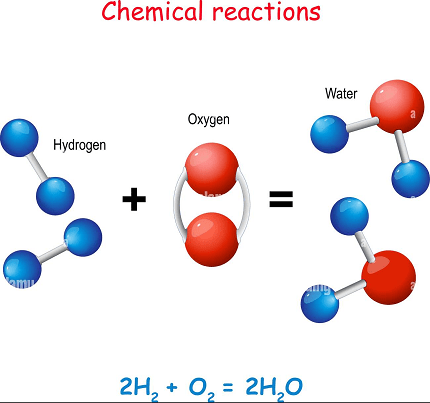
Hydrogen is a highly reactive element that participates in a wide range of chemical reactions. Here are some common reactions involving hydrogen:
- Combustion: When hydrogen gas (H2) is burned in the presence of oxygen gas (O2), it reacts to form water (H2O) and releases energy in the form of heat and light. The chemical equation for this reaction is:
2H2(g) + O2(g) → 2H2O(l) + energy
- Reduction: Hydrogen gas can act as a reducing agent in chemical reactions, meaning it can donate electrons to another substance. For example, in the Haber-Bosch process, hydrogen gas is used to reduce nitrogen gas to produce ammonia (NH3):
N2(g) + 3H2(g) → 2NH3(g)
- Acid-base reactions: Hydrogen ions (H+) can react with other substances to form acidic solutions. For example, when hydrogen chloride gas (HCl) dissolves in water, it ionizes to form hydrogen ions and chloride ions:
HCl(g) + H2O(l) → H3O+(aq) + Cl-(aq)
- Hydrogenation: Hydrogen gas can be used to add hydrogen atoms to unsaturated hydrocarbons (molecules with double or triple bonds) to form saturated hydrocarbons (molecules with only single bonds). For example, in the hydrogenation of vegetable oils, hydrogen gas is used to convert unsaturated fatty acids into saturated fatty acids:
C=C + H2 → C-C
These are just a few examples of the many reactions in which hydrogen can participate.
Reaction (physics)
As depicted by the third of Newton’s laws of movement of traditional mechanics, all powers happen two by two with the end goal that in the event that one item applies a power on another article, the subsequent article applies an equivalent and inverse response force on the first. The third regulation is likewise more by and large expressed as: “To each activity there is constantly gone against an equivalent response: or the shared activities of two bodies upon one another are generally equivalent, and coordinated to opposite parts.” The attribution of which of the two powers is the activity and which is the response is inconsistent. Both of the two can be viewed as the activity, while the other is its related response.
Wikipedia:Reactions to… articles
Wikipedia has a few “Global responses to…” articles that detail the worldwide responses and reactions to a specific episode or occasion. Such articles might be proper when the part of the fundamental article covering reactions and responses arrives at a size that implies the substance can as of now not all squeezed into the primary article and ought to be parted by the rundown style rules.
A few such articles have been erased or consolidated following erasure discusses, for instance due to including unpredictable data or for elevating responses to an occasion past the importance showed by inclusion in dependable sources.
Name reaction
A name response is a synthetic response named after its pioneers or engineers. Among the huge number of natural responses that are known, many such responses are adequately notable to be named after individuals. Notable models incorporate the Grignard response, the Sabatier response, the Wittig response, the Claisen buildup, the Friedel-Specialties acylation, and the Diels-Birch response. Books have been distributed dedicated solely to name responses; the Merck Record, a compound reference book, likewise remembers a supplement for name responses.
As natural science created during the twentieth hundred years, scientific experts began connecting artificially valuable responses with the names of the pioneers or designers; generally speaking, the name is just a memory helper. A few instances of responses that were not exactly found by their namesakes are known. Models incorporate the Pummerer modification, the Pinnick oxidation and the Birch decrease.
Albeit precise methodologies for naming responses in view of the response system or the general change exist (like the IUPAC Terminology for Changes), the more engaging names are in many cases clumsy or not explicit enough, so individuals names are much of the time more functional for productive correspondence.
Nuclear reaction

In atomic physical science and atomic science, an atomic response is a cycle wherein two cores, or a core and an outer subatomic molecule, crash to create at least one new nuclides. Consequently, an atomic response should cause a change of something like one nuclide to another. In the event that a core collaborates with one more core or molecule and they, separate without changing the idea of any nuclide, the cycle is essentially alluded to as a sort of atomic dispersing, as opposed to an atomic response.
On a basic level, a response can include multiple particles impacting, but since the likelihood of at least three cores to meet simultaneously at a similar spot is significantly less than for two cores, such an occasion is incredibly uncommon (see triple alpha interaction for a model exceptionally near a three-body atomic response). The expression “atomic response” may allude either to a change in a nuclide prompted by crash with another molecule or to an unconstrained difference in a nuclide without impact.
Regular atomic responses happen in the collaboration between astronomical beams and matter, and atomic responses can be utilized misleadingly to acquire thermal power, at a customizable rate, on-request. Atomic chain responses in fissionable materials produce actuated atomic parting. Different atomic combination responses of light components power the energy creation of the Sun and stars.
Reversible reaction
A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur simultaneously.
A and B can react to form C and D or, in the reverse reaction, C and D can react to form A and B. This is distinct from a reversible process in thermodynamics.
Weak acids and bases undergo reversible reactions. For example, carbonic acid:
H2CO3 (l) + H2O(l) ⇌ HCO3−(aq) + H3O+(aq).
The concentrations of reactants and products in an equilibrium mixture are determined by the analytical concentrations of the reagents (A and B or C and D) and the equilibrium constant, K. The magnitude of the equilibrium constant depends on the Gibbs free energy change for the reaction. So, when the free energy change is large (more than about 30 kJ mol−1), the equilibrium constant is large (log K > 3) and the concentrations of the reactants at equilibrium are very small. Such a reaction is sometimes considered to be an irreversible reaction, although small amounts of the reactants are still expected to be present in the reacting system. A truly irreversible chemical reaction is usually achieved when one of the products exits the reacting system, for example, as does carbon dioxide (volatile) in the reaction
CaCO3 + 2HCl → CaCl2 + H2O + CO2↑
Reaction rate


The response rate or pace of response is the speed at which a synthetic response happens, characterized as corresponding to the expansion in the convergence of an item for every unit time and to the reduction in the centralization of a reactant for each unit time. Response rates can differ emphatically. For instance, the oxidative rusting of iron under Earth’s air is a sluggish response that can require numerous years, however the ignition of cellulose in a fire is a response that happens in parts of a second. For most responses, the rate diminishes as the response continues. A response’s rate still up in the air by estimating the progressions in fixation over the long haul.
Synthetic energy is the piece of actual science that concerns how paces of substance responses are estimated and anticipated, and how response rate information can be utilized to derive plausible response systems. The ideas of compound energy are applied in many disciplines, like substance designing, enzymology and natural designing.
Nuclear fusion

Atomic combination is a response wherein at least two nuclear cores are consolidated to frame at least one different nuclear cores and subatomic particles (neutrons or protons). The distinction in mass between the reactants and items is appeared as either the delivery or retention of energy. This distinction in mass emerges because of the distinction in atomic restricting energy between the nuclear cores when the response. Atomic combination is the cycle that powers dynamic or primary arrangement stars and other high-greatness stars, where a lot of energy are delivered.
An atomic combination process that produces nuclear cores lighter than iron-56 or nickel-62 will for the most part deliver energy. These components have a somewhat little mass and a generally huge restricting energy per nucleon. Combination of cores lighter than these deliveries energy (an exothermic cycle), while the combination of heavier cores brings about energy held by the item nucleons, and the subsequent response is endothermic. The inverse is valid for the opposite interaction, called atomic splitting. Atomic combination utilizes lighter components, for example, hydrogen and helium, which are overall more fusible; while the heavier components, like uranium, thorium and plutonium, are more fissionable. The super astrophysical occasion of a cosmic explosion can create sufficient energy to combine cores into components heavier than iron.

