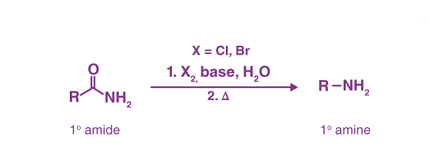
The Hoffmann bromamide degradation is a chemical reaction that involves the conversion of a primary amide to a primary amine by treating it with bromine and sodium or potassium hydroxide. The reaction proceeds via a rearrangement of the intermediate isocyanate to an amine.
The reaction is named after August Wilhelm von Hofmann, who discovered it in 1851. The reaction is used in organic chemistry for the preparation of primary amines from primary amides.
The general reaction scheme for the Hoffmann bromamide degradation is as follows:
RCO-NH2 + Br2 + 3 KOH → RNH2 + K2CO3 + 2 KBr + 2 H2O
The reaction can be carried out under mild conditions, and it is an effective method for the synthesis of primary amines from primary amides. However, the reaction may also lead to the formation of unwanted byproducts, such as secondary and tertiary amines.
Overall, the Hoffmann bromamide degradation is a useful tool in organic chemistry for the preparation of primary amines, but it is important to carefully control the reaction conditions to ensure that the desired product is obtained.
What is Required Reactions: Hoffmann bromamide degradation
The Hoffmann bromamide degradation is a chemical reaction that requires the following reagents and conditions:
- Primary amide: The reaction requires a primary amide as the starting material. Primary amides have the general formula RCONH2, where R is an alkyl or aryl group. The amide should be free from any impurities, such as secondary or tertiary amines, which could lead to unwanted byproducts.
- Bromine: The reaction requires bromine as the brominating agent. Bromine is added dropwise to the primary amide in the presence of a strong base.
- Strong base: The reaction requires a strong base, such as sodium hydroxide (NaOH) or potassium hydroxide (KOH), to facilitate the reaction. The base serves to deprotonate the amide and generate the corresponding isocyanate intermediate.
- Solvent: The reaction requires a solvent to dissolve the amide and facilitate the reaction. Common solvents include water, ethanol, and acetone.
The reaction is typically carried out under mild conditions, such as room temperature or slightly elevated temperatures, and with gentle agitation. The reaction progress can be monitored by TLC or other analytical techniques. The final product, a primary amine, can be isolated by extraction, distillation, or other purification methods.
When is Required Reactions: Hoffmann bromamide degradation
The Hoffmann bromamide degradation is typically used in organic chemistry for the preparation of primary amines from primary amides. It is a useful method for the synthesis of primary amines that are difficult to prepare by other means. The reaction can be used to convert a wide range of primary amides to primary amines, including aliphatic and aromatic amides.
The Hoffmann bromamide degradation is particularly useful in the synthesis of complex molecules, such as natural products and pharmaceuticals, where the presence of a primary amine is required for biological activity or for further functionalization.
The reaction can also be used in the determination of the structure of primary amides. When a primary amide is treated with bromine and a strong base, the reaction proceeds via an isocyanate intermediate. The structure of the amide can be determined by analyzing the structure of the resulting primary amine and the byproducts formed during the reaction.
Overall, the Hoffmann bromamide degradation is a valuable tool in organic chemistry for the preparation of primary amines and for the determination of the structure of primary amides.
Where is Required Reactions: Hoffmann bromamide degradation
The Hoffmann bromamide degradation reaction can be carried out in a laboratory setting using standard laboratory equipment and reagents. The reaction typically requires a primary amide, bromine, a strong base, and a solvent. The reaction can be carried out in a variety of solvents, such as water, ethanol, or acetone, depending on the specific requirements of the reaction.
The reaction vessel can be a round-bottomed flask or a reaction tube, and the reaction can be carried out under mild conditions, such as at room temperature or slightly elevated temperatures, and with gentle agitation.
The progress of the reaction can be monitored by TLC or other analytical techniques, and the final product, a primary amine, can be isolated by extraction, distillation, or other purification methods.
The Hoffmann bromamide degradation reaction is commonly used in organic chemistry laboratories for the preparation of primary amines and the determination of the structure of primary amides. The reaction is also used in the synthesis of complex molecules, such as natural products and pharmaceuticals.
How is Required Reactions: Hoffmann bromamide degradation
The Hoffmann bromamide degradation reaction involves the conversion of a primary amide to a primary amine via the formation of an isocyanate intermediate. The reaction proceeds through the following steps:
- Bromination: The primary amide is treated with bromine in the presence of a strong base, such as sodium or potassium hydroxide. The bromine adds across the carbon-nitrogen double bond in the amide, forming a dibromoamide intermediate.
- Deprotonation: The strong base then deprotonates the nitrogen atom of the dibromoamide, forming an isocyanate intermediate.
- Rearrangement: The isocyanate intermediate undergoes a rearrangement to form a carbamate intermediate, which then decomposes to form the desired primary amine and carbon dioxide. The final product is a primary amine, with the same alkyl or aryl group as the starting amide.
The overall reaction can be represented as follows:
RCO-NH2 + Br2 + 3 KOH → RNH2 + K2CO3 + 2 KBr + 2 H2O
The reaction can be carried out under mild conditions, such as at room temperature or slightly elevated temperatures, and with gentle agitation. The progress of the reaction can be monitored by TLC or other analytical techniques.
The final product, a primary amine, can be isolated by extraction, distillation, or other purification methods, depending on the specific requirements of the reaction. The Hoffmann bromamide degradation reaction is a useful method for the preparation of primary amines and is widely used in organic synthesis.
Production of Reactions: Hoffmann bromamide degradation
The production of the Hoffmann bromamide degradation reaction involves several steps:
- Synthesis of primary amide: The first step involves the synthesis of the primary amide starting material. This can be achieved by reacting an amine with a carboxylic acid or a derivative of a carboxylic acid, such as an acid chloride or an ester. The amide should be of high purity to avoid any side reactions or impurities.
- Bromination: The primary amide is then treated with bromine in the presence of a strong base, such as sodium or potassium hydroxide. The bromine adds across the carbon-nitrogen double bond in the amide, forming a dibromoamide intermediate.
- Deprotonation: The strong base then deprotonates the nitrogen atom of the dibromoamide, forming an isocyanate intermediate.
- Rearrangement: The isocyanate intermediate undergoes a rearrangement to form a carbamate intermediate, which then decomposes to form the desired primary amine and carbon dioxide.
- Isolation and purification: The final product, a primary amine, can be isolated by extraction, distillation, or other purification methods. The product should be purified to remove any remaining impurities and to ensure high purity and yield.
The production of the Hoffmann bromamide degradation reaction requires careful handling of the reagents and a good understanding of the reaction mechanism. The reaction can be scaled up for industrial production, but it is typically carried out on a smaller scale in the laboratory. The reaction can be optimized for specific substrates and conditions to maximize yield and minimize side reactions.
Case Study on Reactions: Hoffmann bromamide degradation
One example of the application of the Hoffmann bromamide degradation reaction is in the synthesis of the anti-cancer drug, vinblastine.
Vinblastine is a natural product that is produced by the Madagascar periwinkle plant. It has potent anti-cancer activity and is used to treat several types of cancer, including Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, and breast cancer. However, the supply of vinblastine from the plant is limited, and its extraction is costly and time-consuming.
In order to overcome this limitation, chemists have developed a synthetic route to vinblastine that involves the use of the Hoffmann bromamide degradation reaction.
The synthesis of vinblastine begins with the synthesis of catharanthine, which is a precursor to vinblastine. Catharanthine is synthesized from tryptamine, an amino acid, through several steps involving reduction, acylation, and cyclization.
The catharanthine is then converted to vindoline by reaction with an aldehyde and a reducing agent. The vindoline is then converted to vinblastine through several steps, including the Hoffmann bromamide degradation reaction.
In the Hoffmann bromamide degradation step, a primary amide is treated with bromine and a strong base to form the desired primary amine. In the case of vinblastine synthesis, the starting material is an N-acetylindole-3-carboxamide, which is converted to N-acetyl-3-methoxy-5-(2-aminoethyl)indole by the Hoffmann bromamide degradation reaction. This intermediate is then further modified to form the vinblastine molecule.
The synthetic route to vinblastine through the Hoffmann bromamide degradation reaction has several advantages over the extraction of vinblastine from the plant. It allows for the production of large quantities of the drug in a more efficient and cost-effective manner. It also allows for the modification of the molecule to improve its pharmacological properties and reduce its side effects.
In conclusion, the Hoffmann bromamide degradation reaction is a useful tool for the synthesis of complex molecules, such as natural products and pharmaceuticals. Its application in the synthesis of vinblastine demonstrates its importance in the development of new drugs and the advancement of medicinal chemistry.
White paper on Reactions: Hoffmann bromamide degradation
Introduction:
The Hoffmann bromamide degradation reaction is a well-known organic reaction that involves the conversion of a primary amide into a primary amine through the use of bromine and a strong base. The reaction was first discovered by August Wilhelm von Hofmann in the mid-19th century and has since been widely used in organic synthesis for the preparation of primary amines, which are important building blocks for the synthesis of pharmaceuticals, agrochemicals, and other organic compounds.
Mechanism:
The mechanism of the Hoffmann bromamide degradation reaction involves several steps. First, the primary amide is treated with bromine in the presence of a strong base, such as sodium or potassium hydroxide. The bromine adds across the carbon-nitrogen double bond in the amide, forming a dibromoamide intermediate. The strong base then deprotonates the nitrogen atom of the dibromoamide, forming an isocyanate intermediate. The isocyanate intermediate undergoes a rearrangement to form a carbamate intermediate, which then decomposes to form the desired primary amine and carbon dioxide.
Applications:
The Hoffmann bromamide degradation reaction has several applications in organic synthesis. It is commonly used for the preparation of primary amines, which are important building blocks for the synthesis of pharmaceuticals, agrochemicals, and other organic compounds. The reaction can also be used for the synthesis of complex natural products, such as the anti-cancer drug, vinblastine.
Advantages:
One of the main advantages of the Hoffmann bromamide degradation reaction is its simplicity and versatility. The reaction can be performed under mild conditions and can be used for the synthesis of a wide range of primary amines. It also allows for the modification of molecules to improve their pharmacological properties and reduce their side effects.
Limitations:
Despite its many advantages, the Hoffmann bromamide degradation reaction has some limitations. One of the main limitations is the requirement for a primary amide starting material. This limits the range of substrates that can be used in the reaction. In addition, the reaction can also produce unwanted side products, such as isocyanates, which can be toxic and difficult to handle.
Conclusion:
The Hoffmann bromamide degradation reaction is an important tool for organic synthesis. Its simplicity and versatility make it an attractive option for the preparation of primary amines and other organic compounds. Despite its limitations, the reaction has found widespread use in the synthesis of pharmaceuticals, agrochemicals, and other important organic compounds. Ongoing research in the field is expected to further improve the reaction’s efficiency and expand its applications.
