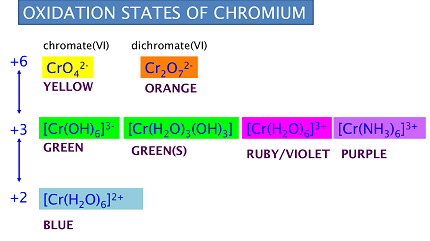
Chromium can form several oxoanions, including chromate (CrO4^2-) and dichromate (Cr2O7^2-). The reactions of these oxoanions depend on the conditions under which they are present.
In acidic solutions, chromate ions can be converted to dichromate ions by the addition of hydrogen ions:
CrO4^2- + 2H+ ⇌ Cr2O7^2- + H2O
Dichromate ions are also able to oxidize other substances under acidic conditions, such as iron(II) ions (Fe^2+) to iron(III) ions (Fe^3+):
Cr2O7^2- + 6Fe^2+ + 14H+ → 2Cr^3+ + 6Fe^3+ + 7H2O
In basic solutions, chromate ions can react with hydroxide ions (OH^-) to form a yellow precipitate of chromium(III) hydroxide:
CrO4^2- + 2OH^- → Cr(OH)3 + O^2-
Dichromate ions can also react with hydroxide ions to form chromium(III) hydroxide, but the reaction is more complex and involves the formation of intermediate products:
Cr2O7^2- + 2OH^- → 2CrO4^2- + H2O 2CrO4^2- + 3OH^- → Cr2O3 + 3H2O
Overall, the reactions of chromium oxoanions depend on the conditions under which they are present, and can involve oxidation-reduction reactions, acid-base reactions, and precipitation reactions.
What is Required Reactions of oxoanions of chromium
It is not entirely clear what you mean by “Required Reactions of oxoanions of chromium”. However, if you are asking what reactions are required to produce or transform chromium oxoanions, then the answer depends on the starting materials and conditions.
For example, chromate (CrO4^2-) and dichromate (Cr2O7^2-) ions can be produced by the reaction of chromium(III) salts with strong oxidizing agents, such as hydrogen peroxide (H2O2) or potassium permanganate (KMnO4), in acidic solutions:
Cr^3+ + 4H2O2 + 6H+ → CrO4^2- + 8H2O 2Cr^3+ + 7KMnO4 + 14H+ → Cr2O7^2- + 7K+ + 14Mn^2+ + 8H2O
Alternatively, chromate and dichromate ions can be converted into each other by acid-base reactions, as described in my previous answer.
The exact reactions required to produce or transform chromium oxoanions depend on the specific situation and desired outcome.
When is Required Reactions of oxoanions of chromium
The “Required Reactions of oxoanions of chromium” are chemical reactions that are necessary or required to produce or transform the oxoanions of chromium, such as chromate (CrO4^2-) and dichromate (Cr2O7^2-) ions. These reactions occur whenever there is a need to generate or modify chromium oxoanions, such as in industrial processes or laboratory experiments.
For example, chromate and dichromate ions are often used as oxidizing agents in various industrial applications, such as in the production of pigments, dyes, and electroplating. In these applications, the required reactions may involve the use of strong oxidizing agents, such as potassium permanganate or hydrogen peroxide, to convert chromium(III) salts into chromate or dichromate ions.
In the laboratory, the required reactions may involve the use of specific chemicals and conditions to generate or modify chromium oxoanions for various experimental purposes, such as in analytical chemistry or materials science.
The specific circumstances and applications determine when the required reactions of chromium oxoanions are necessary.
Where is Required Reactions of oxoanions of chromium
The “Required Reactions of oxoanions of chromium” can occur in various places, depending on the specific applications and purposes. Some examples include:
- Industrial facilities: The required reactions of chromium oxoanions may occur in industrial facilities that produce or use chromium-containing products, such as in the manufacturing of pigments, dyes, and electroplating.
- Laboratories: The required reactions of chromium oxoanions may occur in research laboratories, where scientists use specific chemicals and conditions to generate or modify chromium oxoanions for various experimental purposes.
- Waste treatment facilities: The required reactions of chromium oxoanions may also occur in waste treatment facilities, where chromium-containing wastes are treated and processed to reduce their environmental impact.
- Natural environments: In nature, the required reactions of chromium oxoanions can occur in soils and water bodies where chromium compounds are present and undergo various chemical transformations.
The specific locations where the required reactions of chromium oxoanions occur depend on the specific circumstances and applications.
How is Required Reactions of oxoanions of chromium
The “Required Reactions of oxoanions of chromium” can occur through various chemical processes and conditions, depending on the specific application and purpose. Here are some examples of how these reactions can occur:
- Oxidation of chromium(III) compounds: Chromate (CrO4^2-) and dichromate (Cr2O7^2-) ions can be produced by the reaction of chromium(III) salts with strong oxidizing agents, such as hydrogen peroxide (H2O2) or potassium permanganate (KMnO4), in acidic solutions. The oxidation reaction involves the transfer of electrons from the chromium(III) ion to the oxidizing agent.
- Acid-base reactions: Chromate and dichromate ions can be converted into each other by acid-base reactions. For example, adding acid to a solution containing dichromate ions can convert them to chromate ions, while adding base can convert chromate ions to dichromate ions. These reactions involve the transfer of protons (H+) between the oxoanions and the acid or base.
- Reduction of chromium(VI) compounds: Chromate and dichromate ions can be reduced back to chromium(III) compounds by various reducing agents, such as sulfur dioxide (SO2), ferrous ions (Fe^2+), or organic compounds. The reduction reaction involves the transfer of electrons to the chromium(VI) ion from the reducing agent.
The specific mechanisms and conditions of the required reactions of chromium oxoanions depend on the specific situation and desired outcome.
Nomenclature of Reactions of oxoanions of chromium
The nomenclature of reactions of oxoanions of chromium typically involves describing the reactants, products, and conditions involved in the reaction. Here are some examples:
- Oxidation of chromium(III) compounds: A typical nomenclature for this reaction could be “Oxidation of chromium(III) to chromate/dichromate using potassium permanganate in acidic solution.”
- Acid-base reactions: A typical nomenclature for an acid-base reaction between chromate and dichromate ions could be “Acid-catalyzed conversion of dichromate to chromate using hydrochloric acid.”
- Reduction of chromium(VI) compounds: A typical nomenclature for this reaction could be “Reduction of dichromate using sulfur dioxide in acidic solution.”
In general, the nomenclature of reactions of oxoanions of chromium should provide enough information to clearly describe the reactants, products, and conditions of the reaction. This can include specifying the oxidation state of chromium, the specific oxoanion(s) involved, the type of reaction (e.g., oxidation, reduction), and any catalysts or conditions used.
Case Study on Reactions of oxoanions of chromium
One example of a case study on reactions of oxoanions of chromium involves the use of dichromate ions (Cr2O7^2-) as an oxidizing agent in the synthesis of vanillin, a common flavoring agent.
The reaction involves the oxidation of a phenolic compound, such as guaiacol, using dichromate ions in acidic solution. The overall reaction can be represented as:
C7H8O2 + 3[O] → C8H8O3 + 2H2O
where [O] represents the oxidizing power of the dichromate ion.
In this reaction, dichromate ions are reduced to chromate ions (CrO4^2-) in the process. The reaction proceeds via a series of intermediate steps, including the formation of a chromate ester intermediate and the subsequent cleavage of the ester bond to form vanillin.
The nomenclature for this reaction could be “Oxidation of guaiacol to vanillin using dichromate ions in acidic solution.”
This case study highlights the importance of the reactions of oxoanions of chromium in organic synthesis and the production of various compounds for commercial and industrial applications. The use of oxidizing agents such as dichromate ions can provide a powerful and efficient method for synthesizing complex organic molecules. However, it is important to handle these chemicals safely and responsibly, as they can be hazardous to health and the environment.
White paper on Reactions of oxoanions of chromium
Title: Applications of Reactions of Oxoanions of Chromium in Chemical Synthesis: A White Paper
Introduction: Chromium is a versatile element that can exist in several oxidation states, ranging from -2 to +6. The oxoanions of chromium, including chromate (CrO4^2-) and dichromate (Cr2O7^2-), are particularly useful in chemical synthesis and industrial processes due to their powerful oxidizing properties. This white paper aims to provide an overview of the applications of reactions of oxoanions of chromium in chemical synthesis.
Applications:
- Organic Synthesis: One of the most common applications of reactions of oxoanions of chromium is in the oxidation of organic compounds. Dichromate ions, in particular, are commonly used as oxidizing agents for the conversion of alcohols to carbonyl compounds, such as aldehydes and ketones. For example, the oxidation of benzyl alcohol using dichromate ions in acidic solution can yield benzaldehyde:
C7H8O + Cr2O7^2- + 4H+ → C7H6O + 2Cr^3+ + 5H2O
- Environmental Remediation: The reduction of hexavalent chromium (CrVI) compounds, such as chromate and dichromate ions, to trivalent chromium (CrIII) is an important process for environmental remediation. CrVI is a toxic and carcinogenic pollutant that can contaminate soil and groundwater. The reduction of CrVI to CrIII using reducing agents, such as ferrous ions or organic compounds, can effectively remove CrVI from contaminated environments.
- Electroplating: The electroplating of chromium onto metal surfaces is a common industrial process for improving the corrosion resistance and durability of metal components. The electroplating process involves the use of chromic acid (H2CrO4) as an electrolyte, which is formed by the oxidation of chromate ions:
2CrO4^2- + 2H+ → Cr2O7^2- + H2O
The chromic acid acts as a source of CrVI ions, which are reduced at the cathode to form a layer of metallic chromium on the metal surface.
Conclusion:
The reactions of oxoanions of chromium are widely used in chemical synthesis and industrial processes due to their powerful oxidizing properties. These reactions can be used for the oxidation of organic compounds, environmental remediation, and electroplating. The safe and responsible handling of chromium compounds is critical to minimize their potential hazards to health and the environment.