Oxidation is a type of chemical reaction that involves the loss of electrons or an increase in oxidation state of an atom, ion or molecule. It can occur in a variety of contexts, including combustion reactions, metabolic processes, and corrosion.
In general, oxidation reactions are characterized by the transfer of electrons from a reducing agent to an oxidizing agent. The reducing agent is the species that donates electrons, while the oxidizing agent is the species that accepts electrons.
Some common examples of oxidation reactions include the combustion of fuels such as gasoline or natural gas, which involve the reaction of hydrocarbons with oxygen to produce carbon dioxide and water. Another example is the rusting of iron, which involves the oxidation of iron atoms to form iron oxide.
Oxidation reactions are also important in many biological processes, including cellular respiration, in which glucose is oxidized to produce energy in the form of ATP. Additionally, many drugs and other organic compounds undergo oxidation reactions in the liver as part of the body’s natural detoxification process.
What is Required Aldehydes and Ketones Reactions: Oxidation
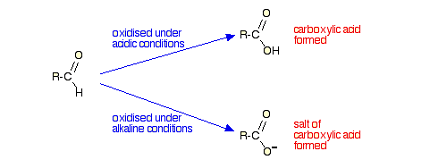
Aldehydes and ketones undergo oxidation reactions in the presence of oxidizing agents such as potassium permanganate (KMnO4), chromium trioxide (CrO3), or nitric acid (HNO3).
Aldehydes are more easily oxidized than ketones, and the reaction typically results in the formation of a carboxylic acid. The oxidation of aldehydes to carboxylic acids is an important reaction in organic chemistry and is commonly used in the laboratory to prepare carboxylic acids from aldehydes.
Ketones, on the other hand, are less reactive and require stronger oxidizing agents to undergo oxidation. The oxidation of ketones typically results in the formation of a dicarbonyl compound, such as a diketone or a ketoacid.
One important example of aldehyde oxidation is the oxidation of ethanol to acetic acid, which is a key step in the production of vinegar. In this reaction, ethanol is first oxidized to acetaldehyde, which is then further oxidized to acetic acid.
Another important example of ketone oxidation is the oxidation of acetone to form acetone peroxide, which is a highly explosive compound that is often used as a terrorist weapon. The oxidation of ketones can also be used in the laboratory to synthesize a variety of different compounds, including cyclic ketones, carboxylic acids, and aldehydes.
When is Required Aldehydes and Ketones Reactions: Oxidation
The oxidation of aldehydes and ketones is a common reaction in organic chemistry and is often used in laboratory synthesis to prepare carboxylic acids, diketones, ketoacids, and other related compounds.
Some specific examples of when aldehyde and ketone oxidation reactions might be required include:
- In the synthesis of carboxylic acids: Aldehydes can be oxidized to carboxylic acids using mild oxidizing agents such as Tollens’ reagent or Fehling’s solution. Ketones, on the other hand, require stronger oxidizing agents such as potassium permanganate or chromium trioxide to undergo oxidation to form carboxylic acids.
- In the production of pharmaceuticals: The oxidation of aldehydes and ketones is an important step in the synthesis of many pharmaceuticals and drug precursors. For example, the oxidation of an aldehyde to a carboxylic acid is a key step in the synthesis of the antihistamine drug loratadine.
- In the preparation of flavors and fragrances: The oxidation of aldehydes and ketones can be used to produce a variety of flavors and fragrances, such as vanilla extract. In this case, vanillin, the compound responsible for the vanilla flavor, is prepared by oxidizing a precursor compound, such as eugenol, using a mild oxidizing agent.
- In biochemical processes: The oxidation of aldehydes and ketones is an important step in several biochemical processes, including the metabolism of carbohydrates and the synthesis of fatty acids. For example, the oxidation of glucose to pyruvate in glycolysis involves several oxidation reactions of aldehydes and ketones.
Where is Required Aldehydes and Ketones Reactions: Oxidation
The oxidation of aldehydes and ketones can occur in various settings, including laboratory synthesis, industrial production, and biochemical processes.
In the laboratory, aldehyde and ketone oxidation reactions are commonly used to prepare a wide range of organic compounds, such as carboxylic acids, diketones, and ketoacids. These reactions typically require the use of specific oxidizing agents and conditions, such as acidic or basic environments, high temperatures, or pressure.
In industrial production, aldehyde and ketone oxidation reactions are used in the synthesis of a variety of chemicals, such as pharmaceuticals, fragrances, and flavors. For example, the oxidation of aldehydes to carboxylic acids is a key step in the production of many important drugs, including antihistamines, antibiotics, and antidepressants.
In biochemical processes, aldehyde and ketone oxidation reactions play a critical role in metabolic pathways, such as glycolysis and the citric acid cycle. These pathways involve the conversion of glucose and other sugars into energy-rich molecules such as ATP, and the oxidation of aldehydes and ketones is an important step in this process.
Overall, the oxidation of aldehydes and ketones is a versatile and important reaction that occurs in a wide range of settings, from laboratory synthesis to industrial production and biochemical processes.
How is Required Aldehydes and Ketones Reactions: Oxidation
The oxidation of aldehydes and ketones typically involves the transfer of electrons from the carbonyl group of the molecule to an oxidizing agent. The reaction is often catalyzed by a metal ion or acid catalyst, which helps to activate the carbonyl group and facilitate the electron transfer.
Aldehydes can be oxidized to carboxylic acids using a variety of oxidizing agents, including Tollens’ reagent, Fehling’s solution, or potassium permanganate. These agents typically contain a metal ion, such as silver or copper, that serves as the electron acceptor in the oxidation reaction. The process involves the transfer of two electrons and two protons from the aldehyde to the oxidizing agent, resulting in the formation of a carboxylic acid.
Ketones, on the other hand, are less reactive and require stronger oxidizing agents, such as chromium trioxide, nitric acid, or potassium permanganate, to undergo oxidation. The oxidation of ketones typically results in the formation of a dicarbonyl compound, such as a diketone or a ketoacid.
The mechanism of ketone oxidation involves the initial formation of an enolate ion, which is then attacked by the oxidizing agent. The enolate ion acts as a nucleophile and attacks the oxidizing agent, transferring electrons to form an intermediate compound. The intermediate compound then undergoes a series of reactions, including proton transfer and reductive elimination, to form the final product.
Overall, the oxidation of aldehydes and ketones is an important reaction in organic chemistry that allows for the synthesis of a wide range of compounds, including carboxylic acids, diketones, ketoacids, and other related compounds. The reaction is typically catalyzed by a metal ion or acid catalyst and involves the transfer of electrons from the carbonyl group of the molecule to an oxidizing agent.
Nomenclature of Aldehydes and Ketones Reactions: Oxidation
The nomenclature of aldehydes and ketones is based on the parent alkane from which the molecule is derived. Aldehydes are named by replacing the -e suffix of the parent alkane with the suffix -al, while ketones are named by replacing the -e suffix of the parent alkane with the suffix -one.
For example, the aldehyde derived from propane is named propanal, while the ketone derived from butane is named 2-butanone.
When aldehydes and ketones undergo oxidation reactions, the resulting products are typically named based on the number and position of the carbonyl groups in the molecule. For example, the oxidation of propanal yields propionic acid, which is named based on the parent alkane propane and the presence of a carboxylic acid functional group. The oxidation of 2-butanone yields 3-hydroxy-2-butanone, which is named based on the position of the hydroxyl group and the presence of the ketone functional group.
In some cases, aldehydes and ketones can undergo further oxidation reactions to form dicarboxylic acids or other more complex compounds. In these cases, the nomenclature of the product is based on the parent alkane and the number and position of the functional groups in the molecule.
Overall, the nomenclature of aldehydes and ketones is straightforward and based on the parent alkane from which the molecule is derived, while the nomenclature of their oxidation products is based on the position and number of the functional groups in the resulting compound.
Case Study on Aldehydes and Ketones Reactions: Oxidation
One example of the importance of aldehydes and ketones oxidation reactions is in the production of vanillin, a flavoring agent commonly used in the food and fragrance industries. Vanillin is typically synthesized from a compound called eugenol, which is found in essential oils from plants such as clove and cinnamon.
The synthesis of vanillin from eugenol involves a series of oxidation reactions, including the oxidation of eugenol to form isoeugenol, and the subsequent oxidation of isoeugenol to form vanillin. The oxidation of isoeugenol to vanillin is a critical step in the synthesis process, and is typically achieved using an oxidizing agent such as potassium permanganate.
The reaction is carried out in a basic solution, and involves the transfer of electrons from the isoeugenol molecule to the oxidizing agent. The resulting product is vanillin, which can be isolated and purified for use in food and fragrance products.
Another example of the importance of aldehyde and ketone oxidation reactions is in the synthesis of pharmaceuticals. For example, the oxidation of aldehydes to carboxylic acids is a key step in the synthesis of many important drugs, including antibiotics, antihistamines, and antidepressants.
One specific example is the synthesis of the drug cefaclor, which is a second-generation cephalosporin antibiotic used to treat a variety of bacterial infections. Cefaclor is synthesized from the aldehyde 7-aminocephalosporanic acid, which is oxidized to form the carboxylic acid precursor for cefaclor.
The oxidation of 7-aminocephalosporanic acid is typically achieved using a strong oxidizing agent such as sodium hypochlorite or potassium permanganate, and the reaction is carried out under basic conditions. The resulting product is then converted into cefaclor through a series of additional chemical reactions.
Overall, aldehydes and ketones oxidation reactions play a critical role in the synthesis of a wide range of compounds, from flavoring agents to pharmaceuticals. These reactions involve the transfer of electrons from the carbonyl group of the molecule to an oxidizing agent, and are typically catalyzed by a metal ion or acid catalyst. The resulting products are named based on the number and position of the functional groups in the molecule, and can be purified and used for a variety of applications.
White paper on Aldehydes and Ketones Reactions: Oxidation
Introduction:
Aldehydes and ketones are important functional groups in organic chemistry, and they undergo a wide range of chemical reactions. One of the most important types of reactions that aldehydes and ketones can undergo is oxidation, which involves the transfer of electrons from the carbonyl group of the molecule to an oxidizing agent. Oxidation reactions are used in the synthesis of a wide range of compounds, from flavoring agents to pharmaceuticals.
Aldehydes Oxidation:
Aldehydes are easily oxidized to form carboxylic acids, which have a carboxyl functional group (-COOH) attached to the carbon atom adjacent to the carbonyl group. The oxidation of aldehydes is typically carried out using a strong oxidizing agent, such as potassium permanganate or chromic acid, under acidic conditions.
For example, the oxidation of ethanol to acetic acid is a common laboratory experiment. Ethanol can be oxidized to acetaldehyde using an oxidizing agent such as pyridinium chlorochromate (PCC) or potassium dichromate in dilute sulfuric acid. The acetaldehyde can then be further oxidized to acetic acid using potassium permanganate under acidic conditions.
Ketones Oxidation:
Ketones are generally more resistant to oxidation than aldehydes, due to the absence of a hydrogen atom on the carbon atom adjacent to the carbonyl group. However, some ketones can be oxidized using strong oxidizing agents such as potassium permanganate or sodium hypochlorite under basic conditions.
For example, the oxidation of cyclohexanone to adipic acid is a key step in the synthesis of nylon. Cyclohexanone can be oxidized to cyclohexanone oxime using hydroxylamine hydrochloride, and the resulting oxime can then be oxidized to adipic acid using hydrogen peroxide and sodium hydroxide.
Applications of Aldehydes and Ketones Oxidation:
Aldehydes and ketones oxidation reactions have numerous applications in industry and academia. One example is in the production of vanillin, a flavoring agent used in the food and fragrance industries. Vanillin is synthesized from eugenol, which is oxidized to form isoeugenol, and the subsequent oxidation of isoeugenol to form vanillin.
Another example is in the synthesis of pharmaceuticals. The oxidation of aldehydes to carboxylic acids is a critical step in the synthesis of many important drugs, including antibiotics, antihistamines, and antidepressants.
Conclusion:
In conclusion, aldehydes and ketones are important functional groups in organic chemistry that undergo a wide range of chemical reactions, including oxidation. The oxidation of aldehydes and ketones is a critical step in the synthesis of a wide range of compounds, from flavoring agents to pharmaceuticals. These reactions involve the transfer of electrons from the carbonyl group of the molecule to an oxidizing agent, and are typically catalyzed by a metal ion or acid catalyst. The resulting products are named based on the number and position of the functional groups in the molecule, and can be purified and used for a variety of applications.
