In chemistry, a reduction reaction is a type of chemical reaction in which electrons are gained by an atom, molecule, or ion. This results in a decrease in the oxidation state or an increase in the number of bonds to hydrogen in the substance being reduced. Reduction reactions can be identified by the addition of electrons, hydrogen atoms, or the removal of oxygen atoms.
Some common examples of reduction reactions include:
- Reduction of metal ions: Metal ions can be reduced to the corresponding metal atoms by the addition of electrons. For example, copper ions can be reduced to copper atoms by the addition of electrons.
- Reduction of carbonyl groups: Carbonyl groups (C=O) can be reduced to alcohols (C-OH) by the addition of hydrogen atoms. For example, acetone can be reduced to isopropanol by the addition of hydrogen atoms.
- Reduction of nitro groups: Nitro groups (NO2) can be reduced to amino groups (NH2) by the addition of hydrogen atoms. For example, nitrobenzene can be reduced to aniline by the addition of hydrogen atoms.
- Reduction of double bonds: Double bonds (C=C) can be reduced to single bonds (C-C) by the addition of hydrogen atoms. For example, ethene can be reduced to ethane by the addition of hydrogen atoms.
Overall, reduction reactions play a significant role in organic and inorganic chemistry and have a wide range of applications in industry and daily life.
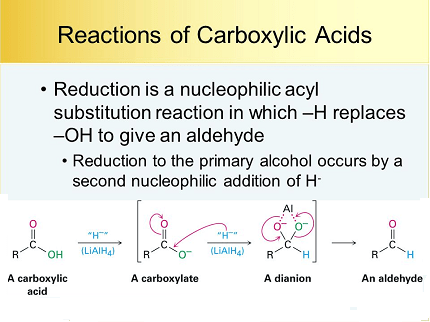
What is Required Carboxylic Acids Reactions: Reduction
The reduction of carboxylic acids is a chemical reaction that involves the conversion of a carboxylic acid functional group (-COOH) into a primary alcohol (-CH2OH) by adding hydrogen atoms to the carbon atom of the carbonyl group. This reaction is known as a carboxylic acid reduction reaction.
There are several methods for reducing carboxylic acids, including:
- Catalytic hydrogenation: This method involves the use of a catalyst, such as palladium, platinum, or nickel, to reduce the carboxylic acid in the presence of hydrogen gas. This reaction typically requires high pressure and high temperature.
- Reduction with Lithium Aluminum Hydride (LiAlH4): This method involves the use of LiAlH4 as a reducing agent to reduce the carboxylic acid to the corresponding alcohol. This reaction is typically carried out in anhydrous ether and can be highly reactive and dangerous if not handled properly.
- Reduction with Borane (BH3) or Borane Dimethyl Sulfide (BH3-SMe2): This method involves the use of borane or borane dimethyl sulfide as a reducing agent to reduce the carboxylic acid to the corresponding alcohol. This reaction is typically carried out in a solvent such as tetrahydrofuran (THF) or diethyl ether.
Overall, the reduction of carboxylic acids is an important reaction in organic chemistry and has many applications in the synthesis of pharmaceuticals, fragrances, and other organic compounds.
When is Required Carboxylic Acids Reactions: Reduction
The reduction of carboxylic acids is a commonly used reaction in organic chemistry that can be used in various applications, such as:
- Synthesis of alcohols: Carboxylic acid reduction reactions can be used to synthesize primary alcohols, which can be used as starting materials for the synthesis of various organic compounds.
- Synthesis of pharmaceuticals: Carboxylic acid reduction reactions can be used in the synthesis of various pharmaceuticals, such as antidepressants, anti-inflammatory drugs, and antihistamines.
- Fragrance industry: Reduction of carboxylic acids can be used in the fragrance industry to synthesize various fragrances and flavorings.
- Production of plasticizers: Reduction of carboxylic acids can be used to produce plasticizers, which are used in the production of plastics to improve their flexibility and durability.
- Synthesis of detergents: Reduction of carboxylic acids can be used in the synthesis of detergents, which are used in cleaning products.
Overall, carboxylic acid reduction reactions are important tools in organic chemistry and have a wide range of applications in various industries.
Where is Required Carboxylic Acids Reactions: Reduction
Carboxylic acids can be reduced to alcohols through a variety of methods. One common method is the use of reducing agents such as lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4). The reduction reaction involves the addition of hydrogen to the carboxylic acid group, resulting in the formation of an alcohol.
Reduction of carboxylic acids can be carried out under various conditions such as in the presence of acid catalysts, metal catalysts, or organic solvents. The choice of reducing agent and conditions will depend on the specific carboxylic acid being reduced and the desired product.
In addition to these methods, carboxylic acids can also be reduced using electrochemical methods, photoredox catalysis, or enzymatic catalysis. These methods offer unique advantages such as mild reaction conditions, selectivity, and sustainability.
The reduction of carboxylic acids is an important reaction in organic synthesis, as it allows for the conversion of carboxylic acids to alcohols, which are versatile intermediates used in the production of various organic compounds.
How is Required Carboxylic Acids Reactions: Reduction
The reduction of carboxylic acids involves the addition of hydrogen to the carboxylic acid functional group, resulting in the formation of an alcohol. The reaction is typically carried out using reducing agents such as lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4).
The general reaction can be written as follows:
R-COOH + 2[H] → R-CH2OH + H2O
where R represents the organic substituent group attached to the carboxylic acid functional group.
The reduction reaction can be carried out under acidic or basic conditions. In acidic conditions, the carboxylic acid is protonated, which makes it more reactive towards the reducing agent. In basic conditions, the reducing agent is activated through deprotonation.
The choice of reducing agent and reaction conditions will depend on the specific carboxylic acid being reduced and the desired product. For example, NaBH4 is a milder reducing agent compared to LiAlH4, and is often used to selectively reduce carboxylic acids in the presence of other functional groups.
Other methods for reducing carboxylic acids include electrochemical methods, photoredox catalysis, and enzymatic catalysis. These methods offer advantages such as mild reaction conditions, selectivity, and sustainability.
Nomenclature of Carboxylic Acids Reactions: Reduction
The nomenclature of carboxylic acids follows the general rules for naming organic compounds. The parent chain of the compound is identified, which is usually the longest carbon chain containing the carboxylic acid functional group. The suffix “-oic acid” is added to the root name of the parent chain to indicate the presence of the carboxylic acid functional group.
For example, the carboxylic acid with a two-carbon chain is called “ethanoic acid”, and the carboxylic acid with a three-carbon chain is called “propanoic acid”.
When carboxylic acids are reduced to alcohols, the suffix “-oic acid” is replaced with “-ol” to indicate the formation of an alcohol. For example, the reduction of ethanoic acid (CH3COOH) would result in the formation of ethanol (CH3CH2OH).
If the carboxylic acid is part of a larger molecule with other functional groups, the carboxylic acid is named as a substituent using the prefix “carboxy-“. For example, in the compound 2-chlorobutanoic acid, the carboxylic acid functional group is attached to the second carbon of a four-carbon chain, and is named as a substituent using the prefix “carboxy-“. The compound is therefore named as 2-carboxybutanoic acid.
In summary, the nomenclature of carboxylic acids follows the general rules for naming organic compounds, with the suffix “-oic acid” added to the root name of the parent chain to indicate the presence of the carboxylic acid functional group. When carboxylic acids are reduced to alcohols, the suffix “-oic acid” is replaced with “-ol” to indicate the formation of an alcohol.
Case Study on Carboxylic Acids Reactions: Reduction
One potential case study on the reduction of carboxylic acids is the conversion of adipic acid to 1,6-hexanediol, a common monomer used in the production of polyesters, polyurethanes, and nylon. Adipic acid is a dicarboxylic acid with the formula HOOC(CH2)4COOH, and can be reduced to 1,6-hexanediol using various reducing agents.
One common method for the reduction of adipic acid to 1,6-hexanediol involves the use of lithium aluminum hydride (LiAlH4) in anhydrous tetrahydrofuran (THF) as the solvent. The reaction is typically carried out at low temperatures under an inert atmosphere to prevent oxidation of the reducing agent.
The general reaction for the reduction of adipic acid to 1,6-hexanediol using LiAlH4 can be written as follows:
HOOC(CH2)4COOH + 4 LiAlH4 → HO(CH2)6OH + 4 LiAl(OH)4
The reaction proceeds through the addition of four equivalents of hydride to the carboxylic acid functional groups of adipic acid, resulting in the formation of 1,6-hexanediol and lithium aluminum tetrahydride (LiAlH4) as a byproduct.
The reduction of adipic acid to 1,6-hexanediol is an important reaction in organic synthesis, as it allows for the production of a versatile monomer used in the production of various polymers and materials. The reaction can be optimized by controlling the reaction conditions, such as the choice of reducing agent, solvent, and temperature, to maximize the yield of the desired product while minimizing the formation of byproducts.
White paper on Carboxylic Acids Reactions: Reduction
Introduction:
Carboxylic acids are an important class of organic compounds that contain a carboxyl functional group (-COOH) attached to a hydrocarbon chain. Reduction of carboxylic acids is an important reaction in organic synthesis, as it allows for the conversion of carboxylic acids to alcohols, which are commonly used in the production of various chemicals and materials. This white paper provides an overview of the reduction of carboxylic acids, including the mechanism of the reaction, commonly used reducing agents, and applications in organic synthesis.
Mechanism:
The reduction of carboxylic acids involves the addition of hydrogen to the carboxyl functional group, resulting in the formation of an alcohol. The reaction is typically carried out using reducing agents such as lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4). The general reaction can be written as follows:
R-COOH + 2[H] → R-CH2OH + H2O
The mechanism of the reduction reaction involves the transfer of hydride ions (H-) from the reducing agent to the carbonyl carbon of the carboxylic acid. The carbonyl carbon is reduced to an alcohol, and the oxygen is protonated to form water. The reaction proceeds through a tetrahedral intermediate, which undergoes protonation and elimination to form the alcohol product.
Commonly Used Reducing Agents:
Lithium aluminum hydride (LiAlH4) and sodium borohydride (NaBH4) are commonly used reducing agents in the reduction of carboxylic acids. LiAlH4 is a stronger reducing agent compared to NaBH4, and is capable of reducing a wide range of functional groups, including carboxylic acids, esters, and ketones. NaBH4 is milder compared to LiAlH4, and is often used to selectively reduce carboxylic acids in the presence of other functional groups.
Applications in Organic Synthesis:
The reduction of carboxylic acids is an important reaction in organic synthesis, as it allows for the conversion of carboxylic acids to alcohols, which are commonly used in the production of various chemicals and materials. For example, the reduction of adipic acid to 1,6-hexanediol is an important reaction in the production of polyesters, polyurethanes, and nylon.
Other applications of the reduction of carboxylic acids include the production of pharmaceuticals, fragrances, and flavors. The reduction of carboxylic acids is often a key step in the synthesis of these compounds, as it allows for the formation of a key intermediate that can be further functionalized to produce the desired product.
Conclusion:
The reduction of carboxylic acids is an important reaction in organic synthesis, as it allows for the conversion of carboxylic acids to alcohols, which are commonly used in the production of various chemicals and materials. The reaction is typically carried out using reducing agents such as lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4), and proceeds through the transfer of hydride ions to the carbonyl carbon of the carboxylic acid. The reduction of carboxylic acids has a wide range of applications in organic synthesis, including the production of polymers, pharmaceuticals, fragrances, and flavors.
