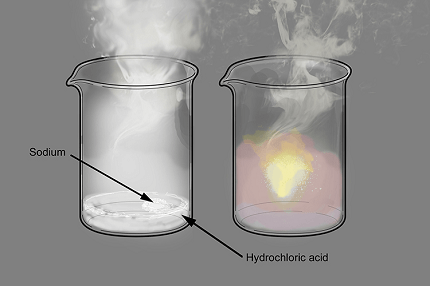
Sodium is a highly reactive alkali metal with the symbol Na and atomic number 11. It has a silvery-white appearance and is soft enough to be cut with a knife. Sodium reacts vigorously with water to produce sodium hydroxide and hydrogen gas. It also reacts with oxygen to form sodium oxide and with halogens to form sodium halides.
Here are some common reactions involving sodium:
- Reaction with water: Sodium reacts vigorously with water to produce hydrogen gas and sodium hydroxide. The reaction is exothermic and can be explosive in the presence of air. The general equation for this reaction is:
2 Na + 2 H2O → 2 NaOH + H2
- Reaction with oxygen: Sodium reacts with oxygen to form sodium oxide. The reaction is highly exothermic and can result in the ignition of the sodium metal. The general equation for this reaction is:
4 Na + O2 → 2 Na2O
- Reaction with chlorine: Sodium reacts with chlorine to form sodium chloride, which is common table salt. The reaction is highly exothermic and can result in the production of a bright yellow flame. The general equation for this reaction is:
2 Na + Cl2 → 2 NaCl
- Reaction with acids: Sodium reacts with acids to produce hydrogen gas and the corresponding sodium salt. For example, when sodium reacts with hydrochloric acid, it produces sodium chloride and hydrogen gas. The general equation for this reaction is:
2 Na + 2 HCl → 2 NaCl + H2
These are just a few examples of the many reactions that sodium can undergo. The reactivity of sodium makes it useful in a wide range of applications, such as in the production of chemicals, alloys, and batteries. However, its highly reactive nature also makes it potentially dangerous, and proper precautions should be taken when handling sodium.
What is Required Alcohols Reactions with: Sodium
When an alcohol reacts with sodium, it undergoes a nucleophilic substitution reaction known as a Williamson ether synthesis. In this reaction, the sodium acts as a strong nucleophile and attacks the electrophilic carbon atom of the alcohol to form an alkoxide intermediate. The alkoxide then reacts with an alkyl halide to produce an ether. The general equation for this reaction is:
ROH + Na → RONa + 1/2H2
RONa + R’-X → ROR’ + NaX
Where ROH is the alcohol, RONa is the alkoxide intermediate, R’-X is the alkyl halide, ROR’ is the ether, and NaX is the sodium halide byproduct.
For example, when sodium reacts with ethanol (CH3CH2OH) and then with methyl iodide (CH3I), it forms methyl ethyl ether (CH3OCH2CH3) and sodium iodide (NaI) as a byproduct:
CH3CH2OH + Na → CH3CH2ONa + 1/2H2
CH3CH2ONa + CH3I → CH3OCH2CH3 + NaI
Overall reaction:
CH3CH2OH + CH3I → CH3OCH2CH3 + HI
This reaction is widely used in organic synthesis to prepare ethers from alcohols. However, care should be taken when handling sodium due to its highly reactive nature.
When is Required Alcohols Reactions with: Sodium
The reaction of alcohols with sodium, also known as the sodium metal reduction of alcohols, is typically used in organic chemistry for the preparation of ethers, alkyl halides, and other organic compounds.
For example, the Williamson ether synthesis, which is the most common reaction involving alcohols and sodium, can be used to synthesize a wide range of ethers. The reaction is often used when there is a need to protect a hydroxyl group (-OH) in an organic molecule, which is a common step in the synthesis of complex organic compounds.
Another use of the reaction between alcohols and sodium is in the synthesis of alkyl halides. This reaction involves the use of a strong alkylating agent, such as an alkyl halide, in combination with sodium metal to convert an alcohol to an alkyl halide.
The reaction of alcohols with sodium can also be used to prepare organosodium compounds, which can be used in a variety of synthetic transformations. Additionally, the reaction can be used to prepare Grignard reagents, which are important in the synthesis of many organic compounds.
Overall, the reaction of alcohols with sodium is an important tool in the arsenal of organic chemists, and it is used in a wide range of synthetic transformations to prepare a variety of organic compounds.
Where is Required Alcohols Reactions with: Sodium
The reaction of alcohols with sodium can be performed in a laboratory setting under controlled conditions. Typically, the reaction is carried out using small pieces of sodium metal and anhydrous solvents, such as diethyl ether or tetrahydrofuran, to prevent the formation of water, which can quench the reaction.
The reaction can be performed using different types of alcohols, including primary, secondary, and tertiary alcohols. The reaction mechanism and conditions depend on the type of alcohol used and the desired product.
The reaction can also be carried out on a larger scale in an industrial setting, although the conditions and safety measures would need to be carefully considered due to the highly reactive nature of sodium.
Overall, the reaction of alcohols with sodium can be performed in both laboratory and industrial settings, and it is an important tool in the synthesis of a wide range of organic compounds.
How is Required Alcohols Reactions with: Sodium
The reaction of alcohols with sodium is typically carried out by adding small pieces of sodium metal to anhydrous solvents containing the alcohol. The reaction is typically exothermic and can be violent, so care must be taken to add the sodium slowly and in small portions.
The reaction mechanism involves the formation of an alkoxide intermediate, which is formed when the sodium metal reacts with the alcohol in the presence of the solvent. The alkoxide intermediate is a strong nucleophile and can react with a variety of electrophiles, including alkyl halides, to form a variety of organic compounds.
One of the most common reactions involving alcohols and sodium is the Williamson ether synthesis, which involves the reaction of an alkoxide intermediate with an alkyl halide to form an ether. The reaction proceeds through an SN2 mechanism and is typically carried out at room temperature in the presence of a catalyst, such as a crown ether.
Another common reaction involving alcohols and sodium is the conversion of alcohols to alkyl halides. This reaction involves the use of a strong alkylating agent, such as an alkyl halide, in combination with sodium metal to convert an alcohol to an alkyl halide. The reaction proceeds through an SN2 mechanism and is typically carried out at low temperatures to minimize side reactions.
The reaction of alcohols with sodium can also be used to prepare organosodium compounds and Grignard reagents, which are important in the synthesis of many organic compounds.
Overall, the reaction of alcohols with sodium is an important tool in the synthesis of a wide range of organic compounds and is typically carried out under carefully controlled conditions to ensure safety and maximize product yields.
Production of Alcohols Reactions with: Sodium
The production of alcohols via reactions with sodium is not a common method for the industrial production of alcohols, as the reaction can be difficult to control and can result in low yields. However, the reaction can be used in a laboratory setting to synthesize alcohols from a variety of starting materials.
One method for the production of alcohols via reactions with sodium is the reduction of alkyl halides or alkyl sulfonates using sodium metal in the presence of an alcohol solvent. The reaction proceeds through a radical mechanism and can be used to prepare primary, secondary, and tertiary alcohols.
Another method for the production of alcohols via reactions with sodium is the reduction of ketones or aldehydes using sodium metal and an alcohol solvent. The reaction proceeds through a nucleophilic addition mechanism and can be used to prepare a variety of secondary and tertiary alcohols.
In both of these methods, the reaction is typically carried out under anhydrous conditions to prevent the formation of water, which can quench the reaction. Care must also be taken to add the sodium slowly and in small portions to avoid the potential for a violent reaction.
Overall, while the production of alcohols via reactions with sodium is not a common industrial method, it can be a useful tool in the synthesis of alcohols in a laboratory setting. The reaction conditions must be carefully controlled to ensure safety and maximize product yields.
Case Study on Alcohols Reactions with: Sodium
One example of the use of alcohols reactions with sodium is in the synthesis of sodium borohydride, which is a commonly used reducing agent in organic chemistry.
Sodium borohydride is prepared by the reaction of sodium metal with boron trioxide in the presence of an alcohol solvent, typically methanol or ethanol. The reaction proceeds through the formation of sodium borate intermediate, which then reacts with hydrogen gas to form sodium borohydride.
Na + B2O3 + 4ROH → 2NaOR + B(OR)3 4NaOR + B(OR)3 + 8H2 → NaBH4 + 3B(OR)3 + 4H2O
In this reaction, the alcohol solvent serves both as a reaction medium and as a source of protons for the formation of the borate intermediate. The reaction is typically carried out at low temperatures and under anhydrous conditions to prevent the formation of water, which can quench the reaction.
Sodium borohydride is a versatile reducing agent and is commonly used in the reduction of aldehydes, ketones, and carboxylic acids, as well as in the reduction of imines and nitriles. The reaction proceeds through the transfer of hydride ions from the sodium borohydride to the carbonyl or imine nitrogen, resulting in the formation of alcohols or amines, respectively.
Overall, the synthesis of sodium borohydride is a key example of the use of alcohols reactions with sodium in the preparation of an important reagent in organic chemistry. The reaction must be carried out under carefully controlled conditions to ensure safety and maximize product yields.
White paper on Alcohols Reactions with: Sodium
Introduction:
Alcohols reactions with sodium are an important tool in organic chemistry for the synthesis of a wide range of organic compounds. Sodium is a highly reactive metal that can react with alcohols to form alkoxide intermediates, which can then be used to carry out a variety of organic reactions. This white paper will explore the various reactions of alcohols with sodium, including their mechanisms, applications, and safety considerations.
Reactions of Alcohols with Sodium:
The reaction of alcohols with sodium proceeds through the formation of an alkoxide intermediate, which is formed when the sodium metal reacts with the alcohol in the presence of a solvent. The alkoxide intermediate is a strong nucleophile that can react with a variety of electrophiles, including alkyl halides, to form a variety of organic compounds.
One of the most common reactions involving alcohols and sodium is the Williamson ether synthesis, which involves the reaction of an alkoxide intermediate with an alkyl halide to form an ether. The reaction proceeds through an SN2 mechanism and is typically carried out at room temperature in the presence of a catalyst, such as a crown ether.
Another common reaction involving alcohols and sodium is the conversion of alcohols to alkyl halides. This reaction involves the use of a strong alkylating agent, such as an alkyl halide, in combination with sodium metal to convert an alcohol to an alkyl halide. The reaction proceeds through an SN2 mechanism and is typically carried out at low temperatures to minimize side reactions.
The reaction of alcohols with sodium can also be used to prepare organosodium compounds and Grignard reagents, which are important in the synthesis of many organic compounds.
Applications:
Alcohols reactions with sodium have a wide range of applications in organic chemistry. The Williamson ether synthesis is commonly used to prepare ethers, which are important solvents and intermediates in organic synthesis. The conversion of alcohols to alkyl halides is an important step in the synthesis of a variety of organic compounds, including pharmaceuticals, agrochemicals, and natural products.
Organosodium compounds and Grignard reagents are important intermediates in organic synthesis and can be used in a variety of reactions, including nucleophilic additions, reductions, and coupling reactions.
Safety Considerations:
The reaction of alcohols with sodium can be highly exothermic and can result in the release of flammable hydrogen gas. Care must be taken to add the sodium slowly and in small portions to avoid the potential for a violent reaction. The reaction should be carried out in a well-ventilated area, and appropriate personal protective equipment, including goggles and gloves, should be worn.
The reaction should be carried out under anhydrous conditions to prevent the formation of water, which can quench the reaction. In addition, the reaction should be carried out in a suitable reaction vessel, such as a round-bottom flask or a pressure-equalizing dropping funnel.
Conclusion:
Alcohols reactions with sodium are an important tool in organic chemistry for the synthesis of a wide range of organic compounds. The reaction proceeds through the formation of an alkoxide intermediate, which can be used to carry out a variety of organic reactions, including the Williamson ether synthesis, the conversion of alcohols to alkyl halides, and the preparation of organosodium compounds and Grignard reagents.
The reaction must be carried out under carefully controlled conditions to ensure safety and maximize product yields. The reaction is typically carried out under anhydrous conditions to prevent the formation of water, which can quench the reaction, and appropriate personal protective equipment should be worn. Overall, alcohols reactions with sodium are an important tool in organic chemistry with a wide range of applications.
