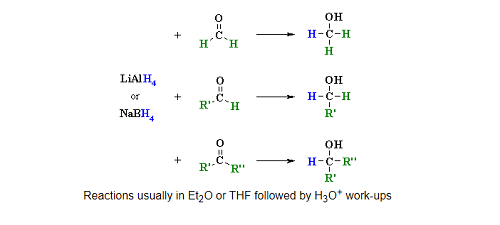
Reduction of aldehydes and ketones is a common reaction in organic chemistry. The most commonly used reducing agents for this purpose are sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4).
Reduction of aldehydes: Aldehydes can be reduced to primary alcohols using either NaBH4 or LiAlH4 in the presence of an acid catalyst such as HCl. The reaction proceeds via the addition of a hydride ion (H-) to the carbonyl group of the aldehyde, followed by protonation of the resulting alkoxide ion.
For example, formaldehyde can be reduced to methanol as follows: HCHO + H- → H2C(OH)- H2C(OH)- + H+ → CH3OH
Reduction of ketones: Ketones can be reduced to secondary alcohols using NaBH4 or LiAlH4. The mechanism is similar to that of aldehyde reduction, involving the addition of a hydride ion to the carbonyl group, followed by protonation.
For example, acetone can be reduced to isopropanol as follows: (CH3)2CO + H- → (CH3)2C(OH)- (CH3)2C(OH)- + H+ → (CH3)2CHOH
It is important to note that LiAlH4 is a stronger reducing agent than NaBH4 and can also reduce carboxylic acids and esters to alcohols, as well as amides to amines. However, LiAlH4 is more reactive and can be dangerous to handle, whereas NaBH4 is milder and safer to use.
What is Required Aldehydes and Ketones Reduction
The reduction of aldehydes and ketones requires a reducing agent such as sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4). These reducing agents are used to add a hydride ion (H-) to the carbonyl group of the aldehyde or ketone, converting it into a primary or secondary alcohol, respectively.
In addition to the reducing agent, an acid catalyst is often used to protonate the resulting alkoxide ion and form the alcohol product. Common acid catalysts include hydrochloric acid (HCl) and acetic acid (CH3COOH).
The reaction is typically carried out in a suitable solvent, such as ethanol or tetrahydrofuran (THF), and at a suitable temperature and pressure. The choice of solvent and reaction conditions will depend on the specific aldehyde or ketone being reduced and the desired product.
Overall, the reduction of aldehydes and ketones is an important synthetic reaction that can be used to prepare a wide range of primary and secondary alcohols, which are useful building blocks in organic chemistry.
When is Required Aldehydes and Ketones Reduction
The reduction of aldehydes and ketones is a common reaction in organic synthesis, and it is used in a variety of contexts where the conversion of a carbonyl group into a primary or secondary alcohol is desired.
Some examples of when the reduction of aldehydes and ketones may be required include:
- Synthesis of alcohols: The reduction of aldehydes and ketones is an important method for the synthesis of primary and secondary alcohols, which are useful building blocks in organic chemistry. This reaction is often used in the preparation of pharmaceuticals, fragrances, and flavors.
- Protection of carbonyl groups: In some cases, aldehydes and ketones can be reduced to alcohols as a protective measure, allowing the carbonyl group to be temporarily removed from the molecule. This can be useful in multi-step synthetic sequences, where a carbonyl group may be reactive or interfere with subsequent reactions.
- Deoxygenation of carbonyl compounds: The reduction of ketones and aldehydes can also be used as a method for deoxygenating carbonyl compounds, producing hydrocarbons or other reduced functional groups.
- Organic reactions: Reduction of aldehydes and ketones is a key step in many organic reactions, such as the Clemmensen reduction and Wolff-Kishner reduction, which are used to convert carbonyl groups into hydrocarbons.
Overall, the reduction of aldehydes and ketones is a versatile reaction that has a wide range of applications in organic chemistry.
Where is Required Aldehydes and Ketones Reduction
The reduction of aldehydes and ketones is a common reaction in organic chemistry and is used in various fields, including:
- Pharmaceutical industry: Reduction of aldehydes and ketones is a key step in the synthesis of many important drugs. For example, the reduction of ketones is a critical step in the synthesis of cholesterol-lowering drugs such as simvastatin and atorvastatin.
- Flavor and fragrance industry: The reduction of aldehydes and ketones is used in the synthesis of many important flavor and fragrance compounds, such as vanillin and dihydrocarvone.
- Polymer industry: The reduction of aldehydes and ketones can be used to modify the properties of polymers. For example, the reduction of aldehydes in polyesters can improve their resistance to hydrolysis.
- Organic chemistry research: The reduction of aldehydes and ketones is a commonly used reaction in organic chemistry research. It is often used to modify functional groups in organic molecules or to synthesize new compounds.
- Biological processes: Reduction of aldehydes and ketones also occurs naturally in biological processes. For example, the conversion of glucose to sorbitol is a reduction reaction that occurs in the human body.
Overall, the reduction of aldehydes and ketones is an important reaction in organic chemistry that has a wide range of applications in various fields, including the pharmaceutical, fragrance, polymer, and research industries.
How is Required Aldehydes and Ketones Reduction
The reduction of aldehydes and ketones typically involves the use of a reducing agent, such as sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4), which donates a hydride ion (H-) to the carbonyl group of the aldehyde or ketone. This reduces the carbonyl group to an alcohol group, forming a primary or secondary alcohol, respectively.
The reaction is often carried out in a suitable solvent, such as ethanol or tetrahydrofuran (THF), and under mild conditions, to avoid over-reduction or other side reactions. The choice of reducing agent and reaction conditions will depend on the specific aldehyde or ketone being reduced and the desired product.
The reduction reaction can be catalyzed by an acid, such as hydrochloric acid (HCl) or acetic acid (CH3COOH), which protonates the resulting alkoxide ion and forms the alcohol product. In some cases, a base catalyst, such as sodium hydroxide (NaOH), may also be used.
The reduction of aldehydes and ketones can be monitored and characterized by various analytical techniques, such as nuclear magnetic resonance (NMR) spectroscopy, infrared (IR) spectroscopy, and gas chromatography (GC).
Overall, the reduction of aldehydes and ketones is a straightforward and useful reaction that can be used to prepare a wide range of primary and secondary alcohols, which are important building blocks in organic chemistry.
Production of Aldehydes and Ketones Reduction
Aldehydes and ketones can be produced in various ways, depending on the starting materials and desired products. Some common methods for producing aldehydes and ketones include:
- Oxidation of alcohols: Primary alcohols can be oxidized to aldehydes, and secondary alcohols can be oxidized to ketones, using various oxidizing agents, such as chromic acid (H2CrO4), pyridinium chlorochromate (PCC), and Jones reagent.
- Dehydration of alcohols: Alcohols can be dehydrated to form alkenes, which can be oxidized further to form aldehydes or ketones, using oxidizing agents such as potassium permanganate (KMnO4) or ozone (O3).
- Friedel-Crafts acylation: Aromatic compounds can be acylated using an acyl chloride or anhydride in the presence of a Lewis acid catalyst, such as aluminum chloride (AlCl3), to produce aromatic ketones.
- Ozonolysis of alkenes: Alkenes can be cleaved by ozone to produce aldehydes or ketones, depending on the substitution pattern of the alkene.
- Grignard reaction: Ketones can be synthesized by reacting a Grignard reagent with an ester or a nitrile, followed by hydrolysis.
Once produced, aldehydes and ketones can be reduced using various reducing agents, as discussed in the previous answer, to produce primary and secondary alcohols.
Overall, the production of aldehydes and ketones is an important step in organic synthesis and can be achieved using a variety of methods, depending on the starting materials and desired products.
Case Study on Aldehydes and Ketones Reduction
One example of the reduction of aldehydes and ketones is the synthesis of menthol, a popular flavoring and fragrance compound found in products such as toothpaste, chewing gum, and cough drops.
Menthol is synthesized from the terpene, limonene, which can be obtained from the peels of citrus fruits. Limonene is first oxidized to form limonene oxide, which is then isomerized to form isopiperitenone, an aldehyde.
Isopiperitenone is then reduced using sodium borohydride (NaBH4) to produce isopiperitenol, a primary alcohol. This alcohol is then oxidized to form the desired menthol product.
The reduction of isopiperitenone to isopiperitenol can be monitored and characterized by various analytical techniques, such as NMR spectroscopy, IR spectroscopy, and GC. The use of NaBH4 as the reducing agent ensures mild conditions and a high yield of the desired product.
This case study demonstrates how the reduction of aldehydes and ketones can be used as a key step in the synthesis of important flavor and fragrance compounds, such as menthol. By carefully selecting the starting material and reducing agent, chemists can achieve high yields and selectivity in this reduction reaction, allowing for the efficient production of desired products.
White paper on Aldehydes and Ketones Reduction
Introduction:
Aldehydes and ketones are important functional groups in organic chemistry, which can be reduced to produce primary and secondary alcohols, respectively. The reduction of aldehydes and ketones is a widely used reaction in organic synthesis, which has various applications in the production of pharmaceuticals, fragrances, and fine chemicals. In this white paper, we will discuss the mechanism, methods, and applications of aldehydes and ketones reduction.
Mechanism of Aldehydes and Ketones Reduction:
The reduction of aldehydes and ketones typically involves the donation of a hydride ion (H-) to the carbonyl group by a reducing agent. This reduces the carbonyl group to an alcohol group, forming a primary or secondary alcohol, respectively. The most commonly used reducing agents for this reaction are sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4), which are mild and selective reducing agents.
The mechanism of NaBH4 reduction of aldehydes and ketones involves the formation of a borohydride ion (BH4-) intermediate, which donates a hydride ion to the carbonyl group. This results in the formation of an alkoxide intermediate, which is protonated by water to form the alcohol product. The overall reaction can be represented as follows:
RCHO + NaBH4 + H2O → RCH2OH + NaBO2 + H2
Similarly, LiAlH4 reduction of aldehydes and ketones involves the formation of an alkoxyaluminum intermediate, which donates a hydride ion to the carbonyl group. The intermediate is then protonated by water to form the alcohol product.
Methods of Aldehydes and Ketones Reduction:
The reduction of aldehydes and ketones can be achieved using various methods, depending on the starting materials and desired products. Some common methods for reducing aldehydes and ketones include:
- NaBH4 reduction: Sodium borohydride is a mild and selective reducing agent, which can be used to reduce aldehydes and ketones to form primary and secondary alcohols, respectively. The reaction can be carried out in a suitable solvent, such as ethanol or THF, under mild conditions to avoid over-reduction or other side reactions.
- LiAlH4 reduction: Lithium aluminum hydride is a more reactive reducing agent than NaBH4, which can reduce a wider range of functional groups, including carboxylic acids and esters. The reaction must be carried out under anhydrous conditions, as LiAlH4 reacts violently with water.
- Catalytic reduction: The reduction of aldehydes and ketones can also be achieved using a metal catalyst, such as platinum, palladium, or nickel, in the presence of a hydrogen source, such as hydrogen gas or a hydrogen donor. This method is often used in industrial applications, as it can be carried out on a large scale.
Applications of Aldehydes and Ketones Reduction:
The reduction of aldehydes and ketones has various applications in organic synthesis and industrial production. Some common applications include:
- Synthesis of alcohols: The reduction of aldehydes and ketones is a key step in the synthesis of primary and secondary alcohols, which are important building blocks in organic chemistry. The resulting alcohols can be further functionalized to produce a wide range of compounds, such as pharmaceuticals, fragrances, and polymers.
- Reduction of carbonyl-containing natural products: Many natural products, such as terpenes and steroids, contain carbonyl groups, which can be reduced to produce useful compounds. For example, the reduction of limonene oxide produces (+)-trans-carveol, a fragrance used in perfumes and flavorings.
- Production of pharmaceuticals: The reduction of aldehydes and ketones is a key step in the synthesis of many pharmaceuticals. For example, the reduction of the ketone group in ibuprofen produces the active ingredient, (S)-(+)-ibuprofen.
- Production of fine chemicals: The reduction of aldehydes and ketones is also used in the production of fine chemicals, such as flavors, fragrances, and agrochemicals. For example, the reduction of benzaldehyde produces benzyl alcohol, which is used as a flavoring agent in foods and beverages.
- Production of polymers: The reduction of aldehydes and ketones is used in the production of polyols, which are important intermediates in the production of polyurethane foams and elastomers. For example, the reduction of acetone produces isopropanol, which can be further converted to isocyanate and reacted with polyols to produce polyurethane products.
Conclusion:
The reduction of aldehydes and ketones is a widely used reaction in organic synthesis, which has various applications in the production of pharmaceuticals, fragrances, and fine chemicals. The most commonly used reducing agents for this reaction are sodium borohydride and lithium aluminum hydride, which are mild and selective reducing agents. The reduction of aldehydes and ketones can be achieved using various methods, such as NaBH4 reduction, LiAlH4 reduction, and catalytic reduction. The resulting alcohols can be further functionalized to produce a wide range of compounds, making this reaction an important tool in organic chemistry.
