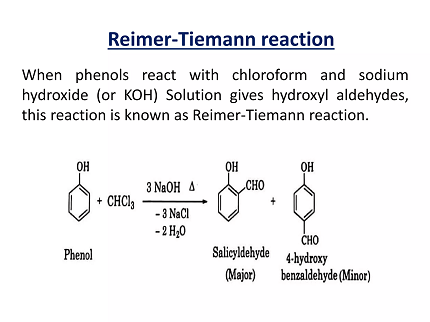
The Reimer-Tiemann reaction is a chemical reaction used to convert phenols into salicylaldehydes or salicylates. The reaction involves the use of chloroform, sodium hydroxide, and a phenol. The mechanism of the reaction involves the formation of a carbonyl intermediate, which is then hydrolyzed to produce the final product.
The reaction proceeds as follows:
- Chloroform (CHCl3) is treated with sodium hydroxide (NaOH) to form the chloroform carbanion (CCl3-).
- The phenol is treated with the chloroform carbanion to form the intermediate, which is a chloroformate ester.
- The intermediate is then hydrolyzed to form the salicylaldehyde or salicylate product.
The Reimer-Tiemann reaction is useful for the synthesis of salicylates, which have a variety of applications in the pharmaceutical and chemical industries. However, the reaction can also produce unwanted side products, such as dichlorobenzenes and trichlorobenzenes, which can be difficult to remove and can be harmful to the environment. Therefore, the reaction should be carefully controlled and monitored to minimize the formation of these byproducts.
What is Required Phenols Reimer-Tiemann reaction
In the Reimer-Tiemann reaction, any phenol with a hydroxyl group (-OH) attached to an aromatic ring can be used as a starting material. The hydroxyl group on the phenol is necessary for the reaction to take place, as it serves as the site of attack for the chloroform carbanion.
The reactivity of the phenol can be influenced by the substituents present on the aromatic ring. For example, phenols with electron-withdrawing substituents, such as nitro (-NO2) or cyano (-CN) groups, are less reactive than phenols with electron-donating substituents, such as methoxy (-OCH3) or alkyl groups. This is because the electron-withdrawing groups reduce the electron density on the aromatic ring, making it less prone to nucleophilic attack.
Overall, any phenol that has a hydroxyl group attached to an aromatic ring can be used in the Reimer-Tiemann reaction. However, the reactivity of the phenol can be influenced by the presence of substituents on the aromatic ring.
When is Required Phenols Reimer-Tiemann reaction
The Reimer-Tiemann reaction is used for the synthesis of salicylaldehydes or salicylates from phenols. Salicylaldehydes and salicylates are important building blocks for the synthesis of many organic compounds, including pharmaceuticals, fragrances, and dyes.
Some specific applications of the Reimer-Tiemann reaction include:
- Synthesis of salicylic acid and its derivatives: Salicylic acid is a key intermediate in the production of aspirin and other analgesic drugs. The Reimer-Tiemann reaction can be used to convert phenol to salicylic acid or its derivatives.
- Synthesis of fragrances: Salicylates are often used as fragrances in perfumes, soaps, and cosmetics. The Reimer-Tiemann reaction can be used to produce these fragrances from phenols.
- Synthesis of dyes: Salicylaldehydes can be used as intermediates in the synthesis of azo dyes. The Reimer-Tiemann reaction can be used to produce these intermediates from phenols.
Overall, the Reimer-Tiemann reaction is a useful tool for the synthesis of salicylaldehydes and salicylates, which have a wide range of applications in various industries.
Where is Required Phenols Reimer-Tiemann reaction
The Reimer-Tiemann reaction can be carried out in a laboratory setting using standard laboratory equipment. The reaction requires a phenol, chloroform, and sodium hydroxide, as well as appropriate solvents and reagents for workup and purification of the product.
The reaction can also be carried out on an industrial scale for the production of salicylates and other organic compounds. In this case, the reaction is typically carried out in large reactors with controlled conditions of temperature, pressure, and reactant concentrations to maximize product yield and minimize byproduct formation.
Overall, the Reimer-Tiemann reaction can be carried out in both laboratory and industrial settings, and its applications are widespread in various industries, including pharmaceuticals, fragrances, and dyes.
How is Required Phenols Reimer-Tiemann reaction
The Reimer-Tiemann reaction is typically carried out by adding a solution of chloroform and sodium hydroxide to a solution of the phenol in an appropriate solvent, such as ethanol or methanol. The reaction is usually carried out under reflux conditions, meaning the mixture is heated at the boiling point with a condenser to prevent the loss of volatile components.
The reaction proceeds through a series of steps, including the formation of a chloroformate intermediate, which then undergoes hydrolysis to form the salicylaldehyde or salicylate product. The mechanism of the reaction involves the attack of the phenoxide ion on the carbene generated from the chloroform, followed by elimination of chloride ion and rearrangement to form the intermediate.
After the reaction is complete, the mixture is typically cooled, and the product is isolated by filtration or extraction. The product may require further purification steps, such as recrystallization or chromatography, to obtain a pure compound.
Overall, the Reimer-Tiemann reaction is a straightforward process that can be carried out in a laboratory setting with standard equipment and techniques. However, careful control of the reaction conditions is required to minimize the formation of byproducts and ensure high product yield and purity.
Nomenclature of Phenols Reimer-Tiemann reaction
In the Reimer-Tiemann reaction, a phenol is typically used as the starting material, which is a type of organic compound that contains an -OH group attached to an aromatic ring. The nomenclature of phenols follows the general rules for naming aromatic compounds, with the addition of the prefix “phenol” to indicate the presence of the hydroxyl group.
The IUPAC name for phenol is “benzene-1-ol”, which indicates that it is a derivative of benzene with a hydroxyl group attached to the first carbon atom. However, the common name “phenol” is widely used in both scientific and industrial settings.
Substituted phenols, which have other groups attached to the aromatic ring in addition to the hydroxyl group, are named based on the position of the substituent relative to the hydroxyl group. The hydroxyl group is always assigned the number 1, and substituents are numbered consecutively around the ring in a manner that gives the lowest possible set of numbers.
For example, 2-methylphenol is commonly known as “o-cresol”, indicating that the methyl group is attached to the ortho (o) position relative to the hydroxyl group. Similarly, 4-chlorophenol is commonly known as “p-chlorophenol”, indicating that the chloro group is attached to the para (p) position relative to the hydroxyl group.
In the context of the Reimer-Tiemann reaction, the starting material phenol is typically referred to simply as “phenol”, with the product being named based on the specific salicylaldehyde or salicylate formed in the reaction.
Case Study on Phenols Reimer-Tiemann reaction
One example of the Reimer-Tiemann reaction in action is the synthesis of salicylic acid from phenol. Salicylic acid is a key intermediate in the production of aspirin and other analgesic drugs.
The reaction proceeds as follows:
- A solution of phenol is prepared in a suitable solvent, such as ethanol or methanol.
- Chloroform and sodium hydroxide are added to the phenol solution, and the mixture is heated under reflux conditions.
- The reaction proceeds to form a chloroformate intermediate, which is hydrolyzed to form salicylic acid and hydrochloric acid.
- The mixture is cooled, and the salicylic acid is isolated by filtration or extraction.
- The product may require further purification steps, such as recrystallization or chromatography, to obtain a pure compound.
The Reimer-Tiemann reaction offers a straightforward and efficient route to the synthesis of salicylic acid from phenol. Salicylic acid can then be converted to aspirin through a series of additional chemical reactions.
This reaction has significant pharmaceutical and industrial applications. Salicylic acid is widely used in the production of aspirin, which is one of the most widely used analgesic drugs in the world. Additionally, salicylic acid is used in the production of a variety of other drugs, as well as in the production of fragrances and dyes.
Overall, the Reimer-Tiemann reaction is an important tool for the synthesis of salicylaldehydes and salicylates, which have a wide range of applications in various industries, including pharmaceuticals, fragrances, and dyes.
White paper on Phenols Reimer-Tiemann reaction
Phenols are a class of organic compounds that contain an aromatic ring with an -OH group attached to it. The Reimer-Tiemann reaction is a classic method for the synthesis of salicylaldehydes and salicylates, which are widely used in the pharmaceutical, fragrance, and dye industries.
The Reimer-Tiemann reaction proceeds through a series of steps, including the formation of a chloroformate intermediate and subsequent hydrolysis to form the product. The reaction is typically carried out under reflux conditions, with careful control of reaction conditions to minimize the formation of byproducts and ensure high product yield and purity.
One notable application of the Reimer-Tiemann reaction is the synthesis of salicylic acid from phenol. Salicylic acid is a key intermediate in the production of aspirin and other analgesic drugs. The reaction is straightforward and efficient, offering a viable route to the synthesis of salicylic acid and its derivatives.
In addition to its pharmaceutical applications, the Reimer-Tiemann reaction has been used in the production of a variety of fragrances and dyes. The reaction can be tailored to produce a range of salicylaldehyde and salicylate derivatives, allowing for the production of compounds with specific functional properties.
Despite its versatility, the Reimer-Tiemann reaction has some limitations. One key issue is the formation of unwanted byproducts, which can reduce the yield and purity of the desired product. Additionally, the reaction can be sensitive to the choice of solvent, requiring careful selection of appropriate solvents to maximize reaction efficiency.
In conclusion, the Reimer-Tiemann reaction is a classic method for the synthesis of salicylaldehydes and salicylates from phenols. The reaction has significant pharmaceutical and industrial applications, but careful control of reaction conditions is required to ensure high product yield and purity.