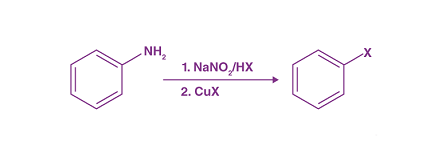
The Sandmeyer reaction is a classic organic chemical reaction that is used to synthesize aryl halides from aryl diazonium salts. The reaction is named after Traugott Sandmeyer, who first reported it in 1884.
Amines can be used as starting materials in the Sandmeyer reaction to synthesize aryl halides bearing amino groups. The reaction proceeds via a diazonium intermediate, which is formed by treating the amine with nitrous acid. The diazonium intermediate then undergoes substitution with a halide ion, typically chloride or bromide, to form the aryl halide product.
The Sandmeyer reaction can also be used to introduce other functional groups onto the aryl ring, such as cyanide or hydroxyl groups, by substituting the halide ion with other nucleophiles. However, the reaction conditions for such transformations may need to be modified to ensure that the desired products are obtained.
What is Required Amines Sandmeyer
To perform the Sandmeyer reaction with amines, the following reagents and conditions are typically required:
- Amine: The amine serves as the starting material for the reaction and should be a primary aromatic amine.
- Nitrous acid (HNO2): This reagent is used to convert the amine to the corresponding diazonium salt intermediate.
- Acid: A strong acid such as hydrochloric acid (HCl) or sulfuric acid (H2SO4) is typically used to generate the nitrous acid in situ and to maintain an acidic environment for the reaction.
- Halide ion source: A source of halide ions, typically sodium or potassium halide, is added to the reaction mixture to facilitate the substitution reaction of the diazonium salt intermediate.
- Temperature control: The reaction is typically performed at low temperatures, around 0-5°C, to prevent unwanted side reactions.
Overall, the Sandmeyer reaction with amines requires careful control of reaction conditions to ensure the selective formation of the desired aryl halide product.
When is Required Amines Sandmeyer
The Sandmeyer reaction with amines is commonly used in organic synthesis for the preparation of aryl halides bearing amino groups. This reaction is particularly useful in the synthesis of compounds such as pharmaceuticals, agrochemicals, and dyes.
For example, the Sandmeyer reaction can be used to synthesize aryl chlorides and bromides from aniline derivatives, which can then be used as starting materials for the synthesis of a variety of bioactive molecules, such as antihypertensive agents, antitumor agents, and antibiotics.
The Sandmeyer reaction can also be used to introduce other functional groups, such as hydroxyl or cyanide groups, onto the aryl ring by substituting the halide ion with other nucleophiles. This allows for the synthesis of a diverse array of functionalized aryl compounds, which have applications in a wide range of fields.
Overall, the Sandmeyer reaction with amines is a versatile and widely used method for the synthesis of aryl halides and other functionalized aryl compounds.
Where is Required Amines Sandmeyer
The Sandmeyer reaction with amines can be performed in a laboratory setting using standard organic chemistry equipment and techniques. The reaction typically involves mixing the amine with an acid and nitrite source, followed by addition of a halide ion source to the reaction mixture at low temperatures. The progress of the reaction can be monitored using various analytical techniques, such as TLC (thin-layer chromatography) or HPLC (high-performance liquid chromatography).
The Sandmeyer reaction can also be performed on a larger scale in an industrial setting for the production of aryl halides and other functionalized aryl compounds. In these cases, specialized equipment and reaction conditions may be used to optimize the reaction yield and purity of the product.
Overall, the Sandmeyer reaction with amines can be carried out in both laboratory and industrial settings, making it a useful method for the synthesis of a variety of organic compounds.
How is Required Amines Sandmeyer
The Sandmeyer reaction with amines involves the following steps:
- Diazotization: The amine is first treated with nitrous acid (HNO2) to generate the corresponding diazonium salt intermediate. The nitrous acid is typically generated in situ by the reaction of a nitrite salt, such as sodium or potassium nitrite, with an acid, such as hydrochloric acid (HCl) or sulfuric acid (H2SO4).
- Halogenation: The diazonium salt intermediate is then reacted with a halide ion source, such as sodium or potassium chloride or bromide, to form the corresponding aryl halide product. The reaction proceeds via an electrophilic aromatic substitution mechanism, where the halide ion replaces the diazonium group on the aryl ring.
The Sandmeyer reaction can be carried out at low temperatures, typically around 0-5°C, to prevent unwanted side reactions, such as decomposition of the diazonium salt. The reaction progress can be monitored using various analytical techniques, such as TLC (thin-layer chromatography) or HPLC (high-performance liquid chromatography).
The Sandmeyer reaction with amines is a useful method for the synthesis of aryl halides and other functionalized aryl compounds, and it can be modified to introduce a wide range of functional groups onto the aryl ring. However, the reaction conditions must be carefully controlled to ensure the selective formation of the desired product.
Production of Amines Sandmeyer
The Sandmeyer reaction with amines is not typically used for the production of amines, but rather for the synthesis of aryl halides and other functionalized aryl compounds from amines. However, amines can be obtained by other methods, such as reduction of nitro compounds or reductive amination of carbonyl compounds.
In the context of the Sandmeyer reaction, amines are used as starting materials for the synthesis of aryl halides. The reaction involves conversion of the amine to the corresponding diazonium salt intermediate, followed by substitution with a halide ion to form the aryl halide product. The reaction can be carried out using a variety of primary aromatic amines, but the choice of amine may affect the reaction yield and selectivity.
Overall, the Sandmeyer reaction with amines is a useful method for the synthesis of aryl halides and other functionalized aryl compounds, but it is not typically used for the production of amines.
Case Study on Amines Sandmeyer
One application of the Sandmeyer reaction with amines is in the synthesis of the drug pramipexole, which is used for the treatment of Parkinson’s disease and restless legs syndrome.
Pramipexole is a dopamine agonist that acts on dopamine receptors in the brain. It has a heterocyclic structure that contains an amino group and a halide substituent. The synthesis of pramipexole involves the Sandmeyer reaction as a key step.
The synthesis of pramipexole via the Sandmeyer reaction with amines can be summarized as follows:
- Diazotization: The amine precursor, 2-amino-6-chloro-4,5,6,7-tetrahydrobenzothiazole, is treated with nitrous acid to generate the corresponding diazonium salt intermediate.
- Halogenation: The diazonium salt intermediate is then reacted with a halide ion source, such as sodium or potassium chloride, to form the corresponding aryl chloride product.
- Reduction: The aryl chloride product is then reduced to the corresponding aryl amine using a reducing agent, such as sodium sulfide.
- Cyclization: The aryl amine is then cyclized to form the heterocyclic ring structure using an acid catalyst, such as hydrochloric acid.
- Further synthetic steps: The resulting product is then subjected to further synthetic steps to introduce additional functional groups and complete the synthesis of pramipexole.
Overall, the Sandmeyer reaction with amines plays a key role in the synthesis of pramipexole and allows for the efficient introduction of a halide substituent onto the heterocyclic ring structure. The synthesis of pramipexole via the Sandmeyer reaction demonstrates the versatility and importance of this reaction in the synthesis of bioactive molecules with complex structures.
White paper on Amines Sandmeyer
Introduction:
The Sandmeyer reaction is a well-known chemical transformation that allows for the synthesis of aryl halides and other functionalized aryl compounds from amines. The reaction involves the conversion of the amine to the corresponding diazonium salt intermediate, followed by substitution with a halide ion to form the aryl halide product. The Sandmeyer reaction with amines has numerous applications in the synthesis of bioactive molecules, materials chemistry, and other areas of chemical research.
In this white paper, we will discuss the principles of the Sandmeyer reaction with amines, its mechanism of action, and its applications in chemical synthesis.
Principles of the Sandmeyer reaction with amines:
The Sandmeyer reaction with amines involves the conversion of a primary aromatic amine to the corresponding diazonium salt intermediate, followed by substitution with a halide ion to form the aryl halide product. The reaction proceeds via an electrophilic aromatic substitution mechanism, where the halide ion replaces the diazonium group on the aryl ring.
The diazonium salt intermediate is generated by treatment of the amine with nitrous acid (HNO2) or a nitrite salt, such as sodium or potassium nitrite, in the presence of an acid, such as hydrochloric acid (HCl) or sulfuric acid (H2SO4). The nitrous acid reacts with the amine to form the diazonium salt intermediate, which is unstable and can decompose to form the corresponding phenol or other side products if not used immediately.
The halide ion source is typically sodium or potassium chloride or bromide, although other halide sources can also be used. The halide ion reacts with the diazonium salt intermediate to form the corresponding aryl halide product. The reaction can be carried out at low temperatures to prevent unwanted side reactions, such as decomposition of the diazonium salt or formation of the corresponding phenol.
Mechanism of the Sandmeyer reaction with amines:
The Sandmeyer reaction with amines proceeds via an electrophilic aromatic substitution mechanism. The diazonium salt intermediate is an electrophile, and the halide ion is a nucleophile. The reaction proceeds as follows:
- Electrophilic attack: The diazonium salt intermediate attacks the aromatic ring, forming an arenium intermediate.
- Halide ion substitution: The halide ion attacks the arenium intermediate, replacing the diazonium group and forming the aryl halide product.
Applications of the Sandmeyer reaction with amines:
The Sandmeyer reaction with amines has numerous applications in chemical synthesis, particularly in the synthesis of bioactive molecules and materials chemistry.
One example of a bioactive molecule synthesized using the Sandmeyer reaction with amines is the drug pramipexole, which is used for the treatment of Parkinson’s disease and restless legs syndrome. Pramipexole has a heterocyclic structure that contains an amino group and a halide substituent, which are introduced using the Sandmeyer reaction with amines.
In materials chemistry, the Sandmeyer reaction with amines can be used to functionalize graphene and other carbon materials with aryl halide groups, which can then be used in a variety of applications, such as catalysis, sensing, and electronics.
Conclusion:
In conclusion, the Sandmeyer reaction with amines is a valuable tool in organic synthesis that enables the conversion of primary aromatic amines to aryl halides and other functionalized aryl compounds. The reaction proceeds via an electrophilic aromatic substitution mechanism and involves the generation of a diazonium salt intermediate, which is subsequently substituted with a halide ion. The Sandmeyer reaction with amines has a broad range of applications in the synthesis of bioactive molecules, materials chemistry, and other areas of chemical research. Overall, the Sandmeyer reaction with amines is a powerful and versatile tool for chemists seeking to functionalize aromatic compounds.
