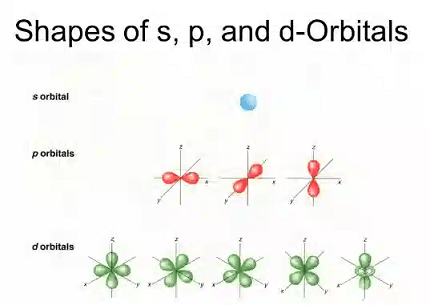
The shapes of s, p, and d orbitals are as follows:
- s orbital: The s orbital is spherically symmetrical, meaning it has a spherical shape. The probability of finding an electron in an s orbital is highest at the nucleus and decreases as you move away from the nucleus.
- p orbital: The p orbital has a dumbbell shape with a node at the nucleus. There are three different p orbitals, each oriented along one of the three axes in space (x, y, and z).
- d orbital: The d orbital has various complex shapes with five different orientations (dxy, dxz, dyz, dx2-y2, and dz2). These shapes are characterized by nodal planes, which are surfaces where the probability of finding an electron is zero.
It’s important to note that the shapes of these orbitals are theoretical representations based on mathematical models, and the actual distribution of electrons in an atom is more complex and dynamic.
What is Required Shapes of s, p and d orbitals
The shapes of s, p, and d orbitals are determined by the solutions of the Schrödinger equation for the hydrogen atom. These solutions represent the wave functions that describe the probability of finding an electron in a particular region of space around the nucleus.
The requirement for s, p, and d orbitals is that they must satisfy the following conditions:
- The wave function must be continuous and finite everywhere in space.
- The wave function must be square integrable, meaning the integral of the square of the wave function over all space must be finite.
- The wave function must satisfy the Schrödinger equation for the hydrogen atom, which describes the behavior of electrons in the atom.
- The wave function must satisfy the Pauli exclusion principle, which states that no two electrons in an atom can have the same set of quantum numbers.
These conditions lead to the specific shapes of s, p, and d orbitals that are observed in atoms. The shapes of the orbitals are determined by the quantum numbers that describe the energy, angular momentum, and magnetic moment of the electrons in the atom.
Who is Required Shapes of s, p and d orbitals
The shapes of s, p, and d orbitals were first proposed by the German physicist Erwin Schrödinger in 1926. Schrödinger developed a wave equation that described the behavior of electrons in atoms and predicted the existence of atomic orbitals with different shapes. His theory, known as wave mechanics or quantum mechanics, revolutionized the field of atomic physics and led to a better understanding of the behavior of electrons in atoms. The shapes of s, p, and d orbitals have since been confirmed experimentally through various spectroscopic techniques.
When is Required Shapes of s, p and d orbitals
The shapes of s, p, and d orbitals are always present in atoms, as they are a fundamental part of the electronic structure of atoms. The specific shapes of these orbitals are determined by the quantum numbers that describe the energy, angular momentum, and magnetic moment of the electrons in the atom. These orbitals play an important role in determining the chemical and physical properties of atoms, such as their reactivity and ability to form chemical bonds. The shapes of s, p, and d orbitals are also essential in understanding the spectroscopic properties of atoms, such as their absorption and emission spectra. So, to summarize, the shapes of s, p, and d orbitals are always present in atoms and are essential for understanding their properties and behavior.
Where is Required Shapes of s, p and d orbitals
The shapes of s, p, and d orbitals refer to the distribution of electron density around the nucleus of an atom. In other words, they describe the regions of space where the probability of finding an electron is highest.
For example, the s orbital has a spherical shape and has the highest electron density at the nucleus, with the electron density decreasing as you move away from the nucleus. The p orbitals have a dumbbell shape, with a node at the nucleus and two lobes of electron density on either side of the node. There are three different p orbitals, each oriented along one of the three axes in space. The d orbitals have more complex shapes and have nodal planes that intersect each other. There are five different d orbitals, each with a different orientation.
The electron density distribution of the s, p, and d orbitals can be represented mathematically using wave functions or electron density maps. These are theoretical representations that describe the probability of finding an electron in a particular region of space. The actual location of an electron in an atom is subject to quantum mechanical uncertainty and cannot be precisely determined.
How is Required Shapes of s, p and d orbitals
The shapes of s, p, and d orbitals are determined by solving the Schrödinger equation for the hydrogen atom, which describes the behavior of electrons in atoms. The Schrödinger equation is a mathematical equation that relates the energy and wave function of an electron in an atom. The wave function of an electron is a complex function that describes the probability of finding an electron in a particular region of space.
The solutions of the Schrödinger equation represent the allowed energy states of electrons in atoms, and each energy state corresponds to a different orbital with a specific shape. The shape of each orbital is determined by the values of the quantum numbers that describe the energy, angular momentum, and magnetic moment of the electron.
The s orbital, for example, has a spherical shape because it has no angular momentum and is spherically symmetric. The p orbitals, on the other hand, have a dumbbell shape because they have angular momentum along a particular axis. The d orbitals have more complex shapes because they have higher angular momentum and nodal planes that intersect each other.
The shapes of s, p, and d orbitals are theoretical representations of the probability of finding an electron in a particular region of space, and they are experimentally observed through various spectroscopic techniques.
Case Study on Shapes of s, p and d orbitals
One example of a case study that illustrates the importance of the shapes of s, p, and d orbitals is the study of transition metal complexes in chemistry.
Transition metal complexes are coordination compounds that consist of a central metal ion surrounded by a group of ligands, which are molecules or ions that donate electrons to the metal ion to form a coordination bond. The electronic and magnetic properties of transition metal complexes are strongly influenced by the shapes of the d orbitals of the metal ion, which determine the orientation and energy of the electrons in the complex.
For example, in octahedral transition metal complexes, the metal ion is surrounded by six ligands that occupy the corners of an octahedron. The d orbitals of the metal ion are split into two sets of three, with one set of three orbitals at higher energy than the other. This splitting is called crystal field splitting and is a result of the electrostatic interactions between the ligands and the metal ion.
The orientation and energy of the electrons in the d orbitals determine the electronic and magnetic properties of the complex. For example, in a low-spin octahedral complex, the electrons preferentially occupy the lower-energy set of three d orbitals, resulting in a weak magnetic moment. In a high-spin octahedral complex, the electrons occupy both sets of d orbitals, resulting in a strong magnetic moment.
The shapes of the d orbitals also influence the reactivity of transition metal complexes. For example, in square planar complexes, the d orbitals of the metal ion are distorted from their normal octahedral shape, which affects the orientation and energy of the electrons in the complex. This can result in different reactivity compared to octahedral complexes.
In summary, the shapes of s, p, and d orbitals are important in understanding the electronic and magnetic properties of transition metal complexes, which are widely used in catalysis, materials science, and biological systems. Understanding the shapes and properties of these orbitals can lead to the development of new and useful compounds with specific electronic and magnetic properties.
White paper on Shapes of s, p and d orbitals
Introduction
The shapes of s, p, and d orbitals are fundamental to our understanding of the electronic structure of atoms and molecules. These orbitals describe the distribution of electron density around the nucleus of an atom and are the basis for our understanding of chemical bonding, reactivity, and molecular properties. This white paper provides an overview of the shapes of s, p, and d orbitals and their importance in chemistry and materials science.
Shapes of s, p, and d orbitals
The s orbital is the simplest orbital and has a spherical shape with the highest electron density at the nucleus. The probability of finding an electron at a distance r from the nucleus is proportional to r^2. The s orbital is spherically symmetric and has no angular momentum. It is the lowest energy orbital and can hold up to two electrons.
The p orbitals have a dumbbell shape with two lobes of electron density on either side of a node at the nucleus. There are three p orbitals, each oriented along one of the three axes in space. The p orbitals have one nodal plane and are higher in energy than the s orbital. They can hold up to six electrons.
The d orbitals have more complex shapes and have nodal planes that intersect each other. There are five different d orbitals, each with a different orientation. The d orbitals have two nodal planes and are higher in energy than the p orbitals. They can hold up to ten electrons.
Importance in chemistry and materials science
The shapes of s, p, and d orbitals play a crucial role in understanding the electronic structure of atoms and molecules, and their importance is particularly evident in chemistry and materials science.
For example, in organic chemistry, the hybridization of atomic orbitals (such as s and p orbitals) determines the geometry of molecules and the strength and directionality of covalent bonds. The shape and orientation of orbitals also influence the reactivity and stability of molecules and can explain phenomena such as stereochemistry and regiochemistry.
In inorganic chemistry, the electronic properties of transition metal complexes are strongly influenced by the shapes of the d orbitals of the metal ion. The orientation and energy of the electrons in the d orbitals determine the electronic and magnetic properties of the complex, and the splitting of the d orbitals (due to the crystal field effect) can result in different electronic and magnetic properties. Understanding the shapes and properties of these orbitals can lead to the development of new and useful compounds with specific electronic and magnetic properties.
In materials science, the electronic and magnetic properties of materials can be related to the shapes of the orbitals involved in bonding. For example, in magnetic materials, the magnetic properties are often related to the orientation of the spin of electrons in partially filled d or f orbitals.
Conclusion
In summary, the shapes of s, p, and d orbitals are fundamental to our understanding of the electronic structure of atoms and molecules. The shape and orientation of orbitals can determine the geometry and reactivity of molecules, the electronic and magnetic properties of transition metal complexes, and the electronic and magnetic properties of materials. The shapes of orbitals are the basis for our understanding of chemical bonding, reactivity, and molecular properties, and they are essential for the development of new and useful compounds and materials.