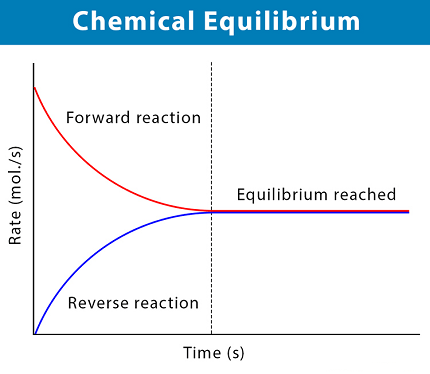
In a substance response, compound harmony is the state where both the reactants and items are available in focuses which have no further propensity to change with time, so there is no discernible change in the properties of the framework. This state results when the forward response continues at a similar rate as the opposite response. The response paces of the forward and in reverse responses are by and large not zero, however they are equivalent. In this way, there are no net changes in the groupings of the reactants and items. Such a state is known as powerful harmony.
Equilibrium chemistry
Balance science is worried about frameworks in substance harmony. The bringing together rule is that the free energy of a framework at harmony is the base conceivable, so the slant of the free energy regarding the response coordinate is zero. This standard, applied to blends at balance gives a meaning of a harmony consistent. Applications incorporate corrosive base, have visitor, metal-complex, dissolvability, parcel, chromatography and redox equilibria.
Equilibrium
A harmony is a condition of a framework where all powers following up on the framework is adjusted. A framework that is in harmony doesn’t change. The word has been utilized for various ideas from various fields of study.
Hydrostatic harmony applies to fluids.
Warm harmony implies as much intensity is entering and leaving something.
Homeostasis is a residing thing keeping its inside balance.
Equilibrioception is the way a creature stands upstanding.
Equilibrium constant
The balance consistent of a substance response is the worth of its response remainder at compound harmony, a state moved toward by a powerful synthetic framework after adequate time has slipped by at which its structure has no quantifiable inclination towards additional change. For a given arrangement of response conditions, the balance steady is free of the underlying logical groupings of the reactant and item species in the combination. In this manner, given the underlying organization of a framework, known balance consistent qualities can be utilized to decide the piece of the framework at harmony. In any case, response boundaries like temperature, dissolvable, and ionic strength may all impact the worth of the balance steady.
An information on balance constants is fundamental for the comprehension of numerous substance frameworks, as well as biochemical cycles, for example, oxygen transport by hemoglobin in blood and corrosive base homeostasis in the human body.
Solidness constants, development constants, restricting constants, affiliation constants and separation constants are a wide range of balance constants.
Thermodynamic equilibrium
Thermodynamic balance is a proverbial idea of thermodynamics. It is an interior condition of a solitary thermodynamic framework, or a connection between a few thermodynamic frameworks associated by pretty much porous or impermeable walls. In thermodynamic balance, there are no net naturally visible progressions of issue nor of energy inside a framework or between frameworks. In a framework that is in its own condition of interior thermodynamic harmony, no perceptible change happens.
Frameworks in shared thermodynamic harmony are at the same time in common warm, mechanical, substance, and radiative equilibria. Frameworks can be in one sort of shared harmony, while not in others. In thermodynamic harmony, a wide range of balance hold without a moment’s delay and endlessly, until upset by a thermodynamic activity. In a perceptible harmony, impeccably or impeccably adjusted minute trades happen; this is the actual clarification of the idea of plainly visible balance.
A thermodynamic framework in a condition of interior thermodynamic harmony has a spatially uniform temperature. Its escalated properties, other than temperature, might be headed to spatial inhomogeneity by a constant long-range force field forced on it by its environmental factors.
In frameworks that are at a condition of non-balance there are, paradoxically, net progressions of issue or energy. In the event that such changes can be set off to happen in a framework in which they are not previously happening, the framework is supposed to be in a meta-stable harmony.
However not a generally named “regulation,” it is a maxim of thermodynamics that there exist conditions of thermodynamic balance. The second law of thermodynamics expresses that when a segregated group of material beginnings from a harmony state, in what bits of it are held at various states by pretty much porous or impermeable parcels, and a thermodynamic activity eliminates or makes the parts more penetrable, then it precipitously arrives at its own new condition of inside thermodynamic balance and this is joined by an expansion in the amount of the entropies of the bits.
Significance Of Research
According to a famous Hudson Maxim, “All progress is born of inquiry. Doubt is often better than overconfidence, for it leads to inquiry, and inquiry leads to invention”. It brings out the significance of research, increased amount of which makes the progress possible. Research encourages scientific and inductive thinking, besides promoting the development of logical habits of thinking and organisation. The role of research in applied economics in the context of an economy or business is greatly increasing in modern times. The increasingly complex nature of government and business has raised the use of research in solving operational problems. Research assumes significant role in the formulation of economic policy for both, the government and business. It provides the basis for almost all government policies of an economic system. Government budget formulation, for example, depends particularly on the analysis of needs and desires of people, and the availability of revenues, which requires research. Research helps to formulate alternative policies, in addition to examining the consequences of these alternatives. Thus, research also facilitates the decision-making of policy-makers, although in itself is not a part of research. In the process, research also helps in the proper allocation of a country’s scarce resources.
Research is also necessary for collecting information on the social and economic structure of an economy to understand the process of change occurring in the country. Collection of statistical information, though not a routine task, involves various research problems. Therefore, large staff of research technicians or experts are engaged by the government these days to undertake this work. Thus, research as a tool of government economic policy formulation involves three distinct stages of operation: investigation of economic structure through continual compilation of facts; (ii) diagnosis of events that are taking place and analysis of the forces underlying them; and (iii) the prognosis i.e., the prediction of future developments (Wilkinson and Bhandarkar, 1979).
Research also assumes significance in solving various operational and planning problems associated with business and industry. In several ways, operations research, market research and motivational research are vital and their results assist in taking business decisions. Market research refers to the investigation of the structure and development of a market for the formulation of efficient policies relating to purchases, production and sales. Operational research relates to the application of logical, mathematical, and analytical techniques to find solution to business problems, such as cost minimization or profit maximization, or the optimization problems. Motivational research helps to determine why people behave in the manner they do with respect to market characteristics. More specifically, it is concerned with the analysis of the motivations underlying consumer behaviour. All these researches are very useful for business and industry, and are responsible for business decision-making.
Research is equally important to social scientists for analyzing the social relationships and seeking explanations to various social problems. It gives intellectual satisfaction of knowing things for the sake of knowledge. It also possesses the practical utility for the social scientist to gain knowledge so as to be able to do something better or in a more efficient manner. The research in social sciences is concerned with both knowledge for its own sake, and knowledge for what it can contribute to solve practical problems.
Case Study on Significance of and chemical equilibrium
Chemical equilibrium is a fundamental concept in chemistry that describes the balance between the forward and reverse reactions of a chemical reaction, in which the concentrations of reactants and products remain constant over time. The equilibrium constant (Kc) is a mathematical expression that describes the relative concentrations of reactants and products at equilibrium and is a measure of the extent of the reaction.
One example of the significance of chemical equilibrium can be seen in the Haber-Bosch process, which is used to produce ammonia (NH3) from nitrogen (N2) and hydrogen (H2) gas. The reaction is exothermic, and the forward reaction is favored at high temperatures, while the reverse reaction is favored at low temperatures. However, a high temperature also leads to a lower yield of ammonia due to the shift towards the reverse reaction.
The Haber-Bosch process is carried out at high pressure (around 200 atm) and moderate temperatures (around 450-500°C) to achieve a reasonable yield of ammonia. The equilibrium constant for the reaction is around 10^5 at these conditions, indicating that the forward reaction is favored at equilibrium. By continuously removing ammonia from the reaction mixture, the reaction can be driven towards the forward direction, leading to a higher yield of ammonia.
Another example of the significance of chemical equilibrium can be seen in acid-base reactions. In water, the autoionization reaction occurs, in which water molecules act as both acids and bases to form hydronium ions (H3O+) and hydroxide ions (OH-). The equilibrium constant for this reaction, known as the ion product constant of water (Kw), is 1.0 x 10^-14 at 25°C.
Acid-base reactions can be characterized by their equilibrium constant, known as the acid dissociation constant (Ka) or the base dissociation constant (Kb), which describe the extent to which an acid or base dissociates in water. For example, the Ka for acetic acid (CH3COOH) is 1.8 x 10^-5, indicating that the acid only partially dissociates in water.
The significance of acid-base equilibrium can be seen in many biological processes, such as the maintenance of pH in blood and the regulation of enzyme activity. The Henderson-Hasselbalch equation, which relates the pH of a solution to the pKa of an acid and the concentration of its conjugate base, is used to predict the behavior of acid-base equilibria in biological systems.
In summary, chemical equilibrium is a fundamental concept in chemistry that describes the balance between the forward and reverse reactions of a chemical reaction. Understanding chemical equilibrium is important for many chemical and biological processes, and the equilibrium constant is a useful tool for predicting the behavior of these systems.
White paper on Significance of and chemical equilibrium
Introduction
Chemical equilibrium is a fundamental concept in chemistry that describes the balance between the forward and reverse reactions of a chemical reaction. In a system at equilibrium, the concentrations of reactants and products remain constant over time. The equilibrium constant (Kc) is a mathematical expression that describes the relative concentrations of reactants and products at equilibrium and is a measure of the extent of the reaction. In this white paper, we will discuss the significance of chemical equilibrium and its applications in various fields.
Applications of Chemical Equilibrium
Haber-Bosch Process
One of the most significant applications of chemical equilibrium is in the Haber-Bosch process, which is used to produce ammonia (NH3) from nitrogen (N2) and hydrogen (H2) gas. The reaction is exothermic, and the forward reaction is favored at high temperatures, while the reverse reaction is favored at low temperatures. However, a high temperature also leads to a lower yield of ammonia due to the shift towards the reverse reaction.
The Haber-Bosch process is carried out at high pressure (around 200 atm) and moderate temperatures (around 450-500°C) to achieve a reasonable yield of ammonia. The equilibrium constant for the reaction is around 10^5 at these conditions, indicating that the forward reaction is favored at equilibrium. By continuously removing ammonia from the reaction mixture, the reaction can be driven towards the forward direction, leading to a higher yield of ammonia.
Acid-Base Equilibria
Acid-base equilibria are another important application of chemical equilibrium. In water, the autoionization reaction occurs, in which water molecules act as both acids and bases to form hydronium ions (H3O+) and hydroxide ions (OH-). The equilibrium constant for this reaction, known as the ion product constant of water (Kw), is 1.0 x 10^-14 at 25°C.
Acid-base reactions can be characterized by their equilibrium constant, known as the acid dissociation constant (Ka) or the base dissociation constant (Kb), which describe the extent to which an acid or base dissociates in water. For example, the Ka for acetic acid (CH3COOH) is 1.8 x 10^-5, indicating that the acid only partially dissociates in water.
The significance of acid-base equilibrium can be seen in many biological processes, such as the maintenance of pH in blood and the regulation of enzyme activity. The Henderson-Hasselbalch equation, which relates the pH of a solution to the pKa of an acid and the concentration of its conjugate base, is used to predict the behavior of acid-base equilibria in biological systems.
Reaction Rates
Chemical equilibrium also plays an important role in reaction rates. The rate of a chemical reaction depends on the concentrations of reactants and products, as well as the activation energy required for the reaction to occur. At equilibrium, the forward and reverse reactions occur at the same rate, resulting in a constant concentration of reactants and products.
However, the rate of a reaction can be affected by changing the conditions of the reaction, such as temperature, pressure, or concentration. By altering these conditions, the equilibrium position can be shifted towards the forward or reverse reaction, leading to changes in the rate of the reaction.
Conclusion
In conclusion, chemical equilibrium is a fundamental concept in chemistry that describes the balance between the forward and reverse reactions of a chemical reaction. Understanding chemical equilibrium is important for many chemical and biological processes, and the equilibrium constant is a useful tool for predicting the behavior of these systems. Applications of chemical equilibrium include the Haber-Bosch process for ammonia production, acid-base equilibria in biological systems, and the regulation of reaction rates.
