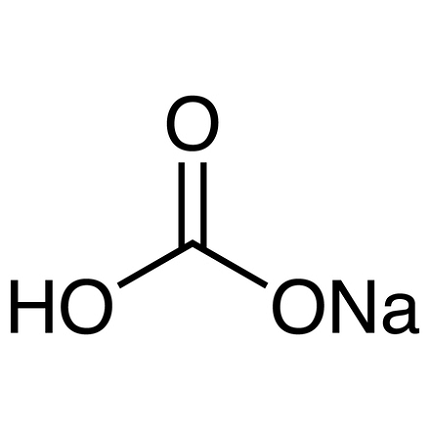
Sodium hydrogen carbonate, also known as sodium bicarbonate or baking soda, is a chemical compound with the molecular formula NaHCO3. It is a white crystalline powder that is soluble in water and has a slightly alkaline taste.
Sodium hydrogen carbonate has a wide range of uses. It is commonly used as a leavening agent in baking, where it reacts with acidic ingredients to produce carbon dioxide gas, which causes dough to rise. It is also used as an antacid to treat heartburn and indigestion, as well as a mild abrasive in cleaning products.
In addition, sodium hydrogen carbonate is used in various industrial processes, including the production of glass, ceramics, and detergents. It can also be used as a fire retardant, and as a component in some types of fire extinguishers.
However, it should be noted that excessive consumption of sodium hydrogen carbonate can lead to side effects such as stomach pain, bloating, and gas. It is also important to avoid inhaling the powder, as it can irritate the lungs.
What is Required Sodium hydrogen carbonate
Sodium hydrogen carbonate (NaHCO3) has several uses in various fields, including:
- Baking: Sodium hydrogen carbonate is widely used in baking as a leavening agent, which helps dough to rise. It reacts with acidic ingredients like buttermilk, yogurt, or lemon juice to produce carbon dioxide gas, which makes the dough or batter rise and gives baked goods a fluffy texture.
- Antacid: Sodium hydrogen carbonate can be used as an antacid to relieve heartburn and indigestion. When ingested, it reacts with stomach acid to neutralize excess acid and alleviate symptoms.
- Cleaning agent: Sodium hydrogen carbonate is mildly abrasive and can be used as a cleaning agent to remove dirt and stains from various surfaces like sinks, toilets, and countertops.
- Fire extinguisher: Sodium hydrogen carbonate is used as a component in some types of fire extinguishers. When heated, it decomposes into carbon dioxide gas, which helps to smother flames.
- Industrial applications: Sodium hydrogen carbonate is used in various industrial processes, including the production of glass, ceramics, and detergents.
- Personal care: Sodium hydrogen carbonate is also used in personal care products like toothpaste and deodorant for its deodorizing and cleansing properties.
It is important to note that while sodium hydrogen carbonate has many beneficial uses, excessive consumption or inhalation of the powder can lead to adverse effects, and it should be used with caution.
Who is Required Sodium hydrogen carbonate
The uses of sodium hydrogen carbonate are diverse and can be applicable to various individuals or industries. Some of the potential users of sodium hydrogen carbonate include:
- Bakers: Sodium hydrogen carbonate is a common leavening agent used in baking, so it is essential for bakers to have access to it.
- Individuals with digestive issues: Sodium hydrogen carbonate is sometimes used as an antacid to relieve heartburn and indigestion, so it may be useful for individuals who suffer from these conditions.
- Cleaning companies: Sodium hydrogen carbonate’s mildly abrasive properties make it an effective cleaning agent, so it may be useful for companies that specialize in cleaning services.
- Industries: Sodium hydrogen carbonate is used in various industrial processes, including the production of glass, ceramics, and detergents, so it may be necessary for companies in these industries to have access to it.
- Firefighters: Sodium hydrogen carbonate is used as a component in some types of fire extinguishers, so it is essential for firefighters to have access to it.
- Personal care companies: Sodium hydrogen carbonate is used in personal care products like toothpaste and deodorant, so it may be useful for companies that produce these types of products.
Overall, the uses of sodium hydrogen carbonate are widespread, and many different individuals and industries may have a need for it.
When is Required Sodium hydrogen carbonate
The uses of sodium hydrogen carbonate can vary depending on the specific application or industry. Here are some examples of when sodium hydrogen carbonate may be required:
- Baking: Sodium hydrogen carbonate is typically required in baking recipes that call for a leavening agent. It is often used in combination with an acidic ingredient, such as buttermilk or lemon juice, to create carbon dioxide gas and help dough or batter rise.
- Antacid: Sodium hydrogen carbonate may be required when someone experiences symptoms of heartburn or indigestion. It can be taken orally to neutralize excess stomach acid and alleviate discomfort.
- Cleaning: Sodium hydrogen carbonate may be required when cleaning surfaces that have stubborn stains or grime. It can be mixed with water to create a paste that is applied to the affected area and then scrubbed away.
- Industrial applications: Sodium hydrogen carbonate may be required in various industrial processes, such as the production of glass or ceramics. It may be added as a component in other chemical reactions or used as a cleaning agent.
- Firefighting: Sodium hydrogen carbonate may be required as a component in some types of fire extinguishers. It can be used to put out fires caused by flammable liquids or electrical equipment.
- Personal care: Sodium hydrogen carbonate may be required in personal care products, such as toothpaste or deodorant. It can help to neutralize odors and provide a mild abrasive for cleaning teeth or skin.
In summary, the required uses of sodium hydrogen carbonate can vary depending on the specific application and industry. It is commonly used in baking, cleaning, and industrial processes, as well as in personal care products and as an antacid.
Where is Required Sodium hydrogen carbonate
The uses of sodium hydrogen carbonate are diverse and can be found in various locations, including:
- Homes: Sodium hydrogen carbonate is commonly found in households for baking and cleaning purposes. It can be purchased at grocery stores, drug stores, and online retailers.
- Bakeries: Sodium hydrogen carbonate is an essential ingredient in many baked goods, so it is commonly used in commercial bakeries.
- Industrial settings: Sodium hydrogen carbonate is used in various industrial processes, including the production of glass, ceramics, and detergents. It may be used in manufacturing facilities or chemical plants.
- Fire departments: Sodium hydrogen carbonate is used as a component in some types of fire extinguishers, so it can be found in fire stations or at the scene of a fire.
- Hospitals and pharmacies: Sodium hydrogen carbonate is sometimes used as an antacid to relieve heartburn and indigestion, so it may be available in hospital settings or pharmacies.
- Personal care stores: Sodium hydrogen carbonate is used in personal care products like toothpaste and deodorant, so it can be found in stores that sell these types of products.
Overall, the locations where sodium hydrogen carbonate can be found depend on the specific application or industry. It is a widely used compound with many different uses, so it can be found in various settings.
How is Required Sodium hydrogen carbonate
Sodium hydrogen carbonate, also known as baking soda or bicarbonate of soda, can be produced through a chemical reaction between sodium chloride, ammonia, and carbon dioxide. Here are the basic steps involved in the production of sodium hydrogen carbonate:
- The first step in producing sodium hydrogen carbonate is to create ammonia gas (NH3) and carbon dioxide gas (CO2) by reacting ammonium chloride (NH4Cl) and calcium carbonate (CaCO3).
- Next, sodium chloride (NaCl) is added to the reaction mixture to create sodium bicarbonate (NaHCO3) and ammonium chloride (NH4Cl).
- The resulting sodium bicarbonate is then separated from the reaction mixture and washed to remove any impurities.
- Finally, the sodium bicarbonate is dried and packaged for use.
Sodium hydrogen carbonate can also be produced through the Solvay process, which involves reacting sodium chloride, ammonia, carbon dioxide, and water. This process is more complex than the first method described above, but it is more commonly used in industrial settings.
In addition to being produced commercially, sodium hydrogen carbonate can also be made at home by mixing baking soda with an acidic ingredient, such as vinegar or lemon juice, to create carbon dioxide gas and water. This reaction can be used as a leavening agent in baking or as a cleaning agent for various household surfaces.
Case Study on Sodium hydrogen carbonate
Case Study: The Role of Sodium Hydrogen Carbonate in Baking
Sodium hydrogen carbonate, also known as baking soda, plays a crucial role in the baking industry. It is a leavening agent that is used to help dough and batter rise, resulting in lighter and fluffier baked goods. In this case study, we will explore the use of sodium hydrogen carbonate in baking and how it affects the quality of the final product.
Background
Baking is a complex process that involves the use of various ingredients and techniques to create a wide range of baked goods. One of the key ingredients in baking is a leavening agent, which is a substance that creates gas and causes dough or batter to rise. There are two types of leavening agents: chemical and biological. Chemical leavening agents, such as sodium hydrogen carbonate, react with other ingredients in the dough or batter to release carbon dioxide gas, causing the mixture to rise. Biological leavening agents, such as yeast, use microorganisms to ferment the sugars in the dough or batter, producing carbon dioxide gas and causing the mixture to rise.
The Use of Sodium Hydrogen Carbonate in Baking
Sodium hydrogen carbonate is a common chemical leavening agent used in baking. It is typically used in recipes that call for an acidic ingredient, such as buttermilk or lemon juice, as the acid reacts with the sodium hydrogen carbonate to produce carbon dioxide gas. The gas creates pockets in the dough or batter, causing it to rise and resulting in a lighter and fluffier texture.
One of the advantages of using sodium hydrogen carbonate as a leavening agent is that it reacts quickly, producing gas as soon as it comes into contact with the acid. This means that bakers can achieve a consistent rise in their baked goods, regardless of how long the dough or batter is left to sit.
However, using too much sodium hydrogen carbonate can lead to an unpleasant taste in the final product. This is because the excess sodium hydrogen carbonate can react with other ingredients, such as the sugar in the recipe, to produce a bitter flavor. Bakers must therefore be careful to use the correct amount of sodium hydrogen carbonate to achieve the desired rise without affecting the taste of the final product.
Conclusion
Sodium hydrogen carbonate is an essential ingredient in the baking industry, used to create a wide range of baked goods, from bread to cakes to cookies. Its role as a chemical leavening agent is critical in achieving a lighter and fluffier texture in the final product. However, bakers must be careful to use the correct amount of sodium hydrogen carbonate to avoid creating an unpleasant taste in the final product.
White paper on Sodium hydrogen carbonate
White Paper: The Science and Applications of Sodium Hydrogen Carbonate
Introduction
Sodium hydrogen carbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a white crystalline powder that is commonly used in a wide range of applications, from baking to cleaning to medical treatments. In this white paper, we will explore the science behind sodium hydrogen carbonate, its properties, and its applications.
Chemical Properties
Sodium hydrogen carbonate is an ionic compound that consists of positively charged sodium ions (Na+) and negatively charged bicarbonate ions (HCO3-). It is a weak base that reacts with acids to produce carbon dioxide gas (CO2), water (H2O), and a salt. The reaction can be represented as follows:
NaHCO3 + HCl → NaCl + H2O + CO2
Sodium hydrogen carbonate is also soluble in water and has a pH of about 8.3 in solution.
Applications
Sodium hydrogen carbonate has a wide range of applications, including:
- Baking: Sodium hydrogen carbonate is a commonly used leavening agent in baking. It reacts with acidic ingredients, such as buttermilk or lemon juice, to produce carbon dioxide gas, which causes the dough or batter to rise and results in a lighter and fluffier texture in the final product.
- Cleaning: Sodium hydrogen carbonate is a mild abrasive that is used in a variety of cleaning products, such as toothpaste and scouring powders. Its alkaline properties make it effective at neutralizing acids and removing stains and odors.
- Medical treatments: Sodium hydrogen carbonate is used in medical treatments for a variety of conditions, including acidosis, heartburn, and indigestion. It works by neutralizing excess acid in the stomach and bloodstream.
- Fire extinguishers: Sodium hydrogen carbonate is also used as an ingredient in some types of fire extinguishers. It reacts with the heat of the fire to produce carbon dioxide gas, which helps to smother the flames.
Safety Considerations
Although sodium hydrogen carbonate is generally considered safe for use in its intended applications, there are some safety considerations to keep in mind. It is a mild irritant to the skin, eyes, and respiratory system and can cause irritation or allergic reactions in some individuals. Ingesting large amounts of sodium hydrogen carbonate can also lead to stomach upset, nausea, and vomiting. As with any chemical compound, it is important to handle sodium hydrogen carbonate with care and follow all safety precautions when using it.
Conclusion
Sodium hydrogen carbonate is a versatile and widely used compound with a variety of applications. Its chemical properties make it effective as a leavening agent in baking, a mild abrasive in cleaning products, and a neutralizer in medical treatments. While it is generally considered safe for use, it is important to handle sodium hydrogen carbonate with care and follow all safety precautions when using it.