Specific heat is the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius (or one Kelvin) without any change in phase. There are two types of specific heat:
- Specific heat at constant pressure (Cp): This is the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius while keeping the pressure constant.
- Specific heat at constant volume (Cv): This is the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius while keeping the volume constant.
The specific heat depends on the nature of the substance and its physical state (solid, liquid, or gas) and can be measured experimentally. The specific heat is usually expressed in units of joules per gram per degree Celsius (J/g°C) or joules per mole per degree Celsius (J/mol°C).
The specific heat of a substance is an important property as it helps in calculating the amount of heat required to raise or lower the temperature of a substance during a chemical or physical change. It also helps in understanding the heat transfer processes involved in various engineering applications.
What is Required Specific heats
Required specific heats are specific heats that are needed to calculate the amount of heat required to change the temperature of a substance during a chemical or physical process where the pressure and/or volume changes.
When the pressure and/or volume of a substance changes during a process, the specific heat of the substance also changes. In this case, two different specific heats are required:
- Enthalpy-specific heat at constant pressure (Cp): This specific heat is used to calculate the heat required to change the temperature of a substance while the pressure is kept constant.
- Internal energy-specific heat at constant volume (Cv): This specific heat is used to calculate the heat required to change the temperature of a substance while the volume is kept constant.
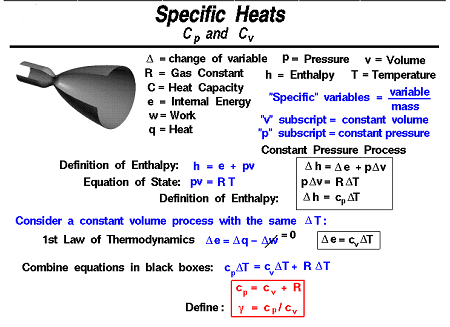
The enthalpy-specific heat and internal energy-specific heat are related by the following equation:
Cp – Cv = R
where R is the gas constant.
The required specific heats are often tabulated for different substances at various temperatures and pressures. They are important in many fields, such as thermodynamics, chemical engineering, and material science, where the precise calculation of heat transfer and energy consumption is essential.
When is Required Specific heats
Required specific heats are used when there is a change in pressure and/or volume of a substance during a chemical or physical process. In such cases, the specific heat of the substance changes, and the required specific heats are needed to calculate the amount of heat required to change the temperature of the substance.
For example, consider the process of heating a gas in a piston-cylinder assembly where the gas undergoes a change in volume. The heat required to raise the temperature of the gas depends on the specific heat of the gas, which changes as the volume changes. In this case, the required specific heats, namely the enthalpy-specific heat at constant pressure (Cp) and internal energy-specific heat at constant volume (Cv), are needed to calculate the amount of heat required to raise the temperature of the gas.
Required specific heats are also used in various other processes, such as heating or cooling a liquid or solid under different pressures or volumes, heating or cooling a gas during a chemical reaction, and calculating the energy requirements for chemical processes in chemical engineering. Therefore, the use of required specific heats is essential for understanding and predicting the thermal behavior of substances under different conditions.
Where is Required Specific heats
Required specific heats can be found in thermodynamics textbooks, engineering handbooks, and reference tables of thermodynamic properties of substances. These tables provide enthalpy-specific heat at constant pressure (Cp) and internal energy-specific heat at constant volume (Cv) values for different substances at various temperatures and pressures.
The National Institute of Standards and Technology (NIST) provides a comprehensive database of thermodynamic properties for various substances, including required specific heats. The NIST Chemistry WebBook is a free online resource that provides access to these properties and allows users to search for specific substances and conditions.
Many engineering software programs and simulation tools also include databases of thermodynamic properties, including required specific heats. These tools allow engineers and scientists to simulate and predict the behavior of substances under different conditions and perform calculations involving heat transfer, energy consumption, and other thermodynamic properties.
In summary, required specific heats can be found in reference tables, handbooks, and databases of thermodynamic properties of substances. They are essential for understanding and predicting the thermal behavior of substances under different conditions in a variety of fields, including engineering, chemistry, and material science.
How is Required Specific heats
Required specific heats are typically determined experimentally using calorimetry or other thermal analysis techniques. In calorimetry, the heat exchange between a system and its surroundings is measured, and from this information, the specific heat of the system can be calculated.
For enthalpy-specific heat at constant pressure (Cp), the experiment is conducted at constant pressure, and the heat exchanged is measured while the temperature of the substance is raised. The heat capacity at constant pressure, Cp, is then calculated as the heat exchanged divided by the mass of the substance and the temperature change.
For internal energy-specific heat at constant volume (Cv), the experiment is conducted at constant volume, and the heat exchanged is measured while the temperature of the substance is raised. The heat capacity at constant volume, Cv, is then calculated as the heat exchanged divided by the mass of the substance and the temperature change.
In addition to experimental methods, required specific heats can also be estimated using theoretical models and correlations. For example, the Debye model and Einstein model are two theoretical models used to calculate the specific heat of solids. Empirical correlations can also be used to estimate required specific heats of liquids and gases based on experimental data.
Once the required specific heats are determined, they can be tabulated and used in various engineering and scientific applications to calculate the amount of heat required to change the temperature of a substance under different conditions.
Nomenclature of Specific heats
The nomenclature of specific heats can vary depending on the context and the specific field of study. However, in general, the symbol used to represent specific heat is “C,” and it is usually accompanied by a subscript indicating the conditions under which the specific heat is measured.
The two most common subscripts used for specific heats are “p” and “v,” which represent the conditions of constant pressure and constant volume, respectively. Therefore, the enthalpy-specific heat at constant pressure is denoted as Cp, and the internal energy-specific heat at constant volume is denoted as Cv.
In some cases, specific heats may also be measured at other conditions, such as constant entropy (Cs), constant temperature (Ct), or adiabatic conditions (Ca). In such cases, the subscript used to denote the condition will vary accordingly (i.e., Cs, Ct, Ca, etc.).
It is important to note that the units of specific heat depend on the units used to express the heat and temperature. The most commonly used units for specific heat are joules per gram-Kelvin (J/g-K) and calories per gram-Kelvin (cal/g-K). However, other units such as joules per mole-Kelvin (J/mol-K) or kilojoules per kilogram-Kelvin (kJ/kg-K) may also be used, depending on the specific application.
Overall, the nomenclature of specific heats follows a general convention of using the symbol “C” with a subscript to indicate the conditions under which the specific heat is measured.
Case Study on Specific heats
One example of a case study involving specific heats is the design of a heat exchanger used for cooling a process stream in a chemical plant. In this scenario, the specific heats of both the process fluid and the cooling fluid are important parameters that need to be considered in the design and operation of the heat exchanger.
The process fluid, which is a liquid mixture containing various components, enters the heat exchanger at a high temperature and must be cooled to a specified temperature for downstream processing. The cooling fluid, which is typically water or another coolant, flows through the heat exchanger and absorbs the heat from the process fluid, thereby lowering its temperature.
To design the heat exchanger, the required heat transfer area, flow rates, and temperature differences must be calculated based on the specific heats of the two fluids. The enthalpy-specific heat at constant pressure (Cp) of the process fluid and the cooling fluid must be known to determine the amount of heat exchanged during the cooling process. The specific heat of the process fluid may vary depending on the composition of the mixture, so it may be necessary to use correlations or experimental data to estimate this parameter.
In addition to the specific heats, other factors such as pressure drops, fouling, and corrosion must also be considered in the design and operation of the heat exchanger. The design must be optimized to minimize the energy consumption while ensuring that the cooling requirements are met.
Once the heat exchanger is designed and installed, it must be monitored and maintained to ensure that it is operating at peak efficiency. Regular cleaning, inspection, and repair may be necessary to prevent fouling or corrosion that could impact the heat transfer efficiency and the overall performance of the heat exchanger.
In summary, the specific heats of process fluids and cooling fluids play a critical role in the design and operation of heat exchangers and other thermal systems. By accurately measuring or estimating these parameters, engineers and operators can optimize the performance of these systems and ensure that they operate safely and efficiently.
White paper on Specific heats
Introduction:
Specific heats are important thermodynamic properties that describe the amount of heat energy required to raise the temperature of a substance by one degree Celsius or Kelvin. Specific heats can be used to calculate the amount of heat required to change the temperature of a substance, and they are important parameters in many engineering and scientific applications, such as the design of heat exchangers, the analysis of thermodynamic processes, and the calculation of energy balances.
This white paper provides an overview of specific heats, including their definition, units of measurement, and applications in various fields of study.
Definition:
Specific heat is defined as the amount of heat energy required to raise the temperature of a unit mass of a substance by one degree Celsius or Kelvin. The specific heat of a substance depends on its physical and chemical properties, such as its molecular structure, density, and phase (solid, liquid, or gas).
There are two types of specific heats: enthalpy-specific heat at constant pressure (Cp) and internal energy-specific heat at constant volume (Cv). Enthalpy-specific heat at constant pressure is the amount of heat energy required to raise the temperature of a substance at constant pressure, while internal energy-specific heat at constant volume is the amount of heat energy required to raise the temperature of a substance at constant volume.
Units of measurement:
The units of measurement for specific heat depend on the units used to express the heat energy and temperature. The most commonly used units for specific heat are joules per gram-Kelvin (J/g-K) and calories per gram-Kelvin (cal/g-K). Other units, such as joules per mole-Kelvin (J/mol-K) or kilojoules per kilogram-Kelvin (kJ/kg-K), may also be used, depending on the specific application.
Applications:
Specific heats have a wide range of applications in various fields of study, including:
- Thermodynamics: Specific heats are used to calculate the energy required to change the temperature of a substance during various thermodynamic processes, such as heating, cooling, compression, and expansion.
- Heat transfer: Specific heats are used to calculate the amount of heat transferred during various heat transfer processes, such as conduction, convection, and radiation.
- Engineering: Specific heats are used in the design and operation of various engineering systems, such as heat exchangers, boilers, turbines, and engines.
- Chemistry: Specific heats are used to calculate the energy required to change the temperature of chemical reactions and to determine the thermodynamic properties of chemical compounds.
Conclusion:
Specific heats are important thermodynamic properties that describe the amount of heat energy required to raise the temperature of a substance by one degree Celsius or Kelvin. They have a wide range of applications in various fields of study, including thermodynamics, heat transfer, engineering, and chemistry. Accurate measurement or estimation of specific heats is critical for the design and operation of thermal systems and the calculation of energy balances.
