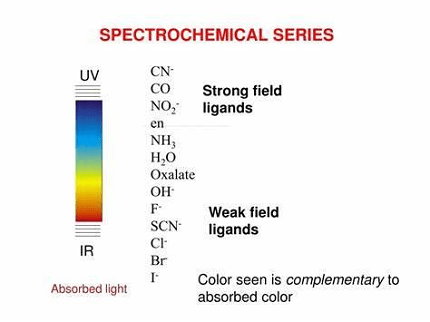
The spectrochemical series is a list of ligands arranged in order of their ability to cause splitting of d-orbitals in a metal ion complex. The series is important in coordination chemistry and helps to predict the electronic properties and reactivity of metal complexes.
The spectrochemical series is as follows, from weakest to strongest field ligands:
I- < Br- < S2- < SCN- < Cl- < NO3- < N3- < F- < OH- < C2O4 2- < H2O < NCS- < CH3CN < pyridine < NH3 < en < bipy < phen < NO2- < PPh3 < CN-
In general, ligands towards the right of the series (e.g. CN-) are stronger field ligands and cause larger splittings of d-orbitals in a metal ion complex. This leads to a lower crystal field splitting energy (CFSE) and a more high-spin character in the complex. Ligands towards the left of the series (e.g. OH-) are weaker field ligands and cause smaller splittings of d-orbitals in a metal ion complex, leading to a higher CFSE and a more low-spin character in the complex.
What is Required Spectrochemical series
The Required Spectrochemical series is a list of ligands arranged in order of their ability to cause splitting of d-orbitals in a metal ion complex, based on a specific metal ion. The series is important in predicting the electronic properties and reactivity of metal complexes with a particular metal ion.
For example, the Required Spectrochemical series for octahedral complexes of the first-row transition metal ions in their +2 oxidation state, based on the spectrochemical series of ligands in general, is as follows, from weakest to strongest field ligands:
I- < Br- < S2- < SCN- < Cl- < NO3- < N3- < F- < OH- < H2O < pyridine < NH3 < en < bipy < phen < CN-
Note that in this series, ligands like C2O4 2- and PPh3 have been removed, as they are not common ligands for octahedral complexes of the first-row transition metal ions in their +2 oxidation state.
In general, ligands towards the right of the series (e.g. CN-) are stronger field ligands and cause larger splittings of d-orbitals in a metal ion complex. This leads to a lower crystal field splitting energy (CFSE) and a more high-spin character in the complex. Ligands towards the left of the series (e.g. OH-) are weaker field ligands and cause smaller splittings of d-orbitals in a metal ion complex, leading to a higher CFSE and a more low-spin character in the complex.
When is Required Spectrochemical series
The Required Spectrochemical series is used in coordination chemistry when predicting the electronic properties and reactivity of metal complexes with a specific metal ion. The series is typically used for octahedral complexes of first-row transition metal ions in their +2 oxidation state.
The Required Spectrochemical series is important because the strength of the ligand field determines the electronic structure of the metal complex, including the number of unpaired electrons in the d-orbitals. This, in turn, influences the physical and chemical properties of the complex, such as its magnetic and spectral properties, as well as its reactivity.
By knowing the relative strengths of different ligands for a particular metal ion, chemists can predict the properties of metal complexes formed with those ligands. The Required Spectrochemical series provides a useful tool for predicting the properties of metal complexes and for selecting ligands with desirable properties for specific applications in areas such as catalysis, sensing, and medicine.
Where is Required Spectrochemical series
The Required Spectrochemical series is a theoretical concept used in coordination chemistry and is not a physical location. It is a list of ligands arranged in order of their ability to cause splitting of d-orbitals in a metal ion complex, based on a specific metal ion.
The Required Spectrochemical series is typically used in the laboratory by chemists when designing and synthesizing metal complexes with specific properties. It is also used in computational chemistry to predict the properties of metal complexes and to guide the design of new ligands.
The Required Spectrochemical series is a fundamental concept in coordination chemistry and is widely used in research, as well as in industrial applications such as catalysis, materials science, and medicinal chemistry.
How is Required Spectrochemical series
The Required Spectrochemical series is determined by the relative strengths of the ligand field created by different ligands around a particular metal ion in a complex. The strength of the ligand field determines the extent of splitting of the d-orbitals of the metal ion, which in turn influences the electronic structure and properties of the complex.
To determine the Required Spectrochemical series for a particular metal ion, a set of ligands are chosen and their ability to cause splitting of d-orbitals in the metal ion complex is measured experimentally or calculated theoretically. The resulting data is then used to rank the ligands in order of their ability to cause splitting of the d-orbitals, creating the Required Spectrochemical series.
The spectrochemical series in general can be used as a starting point to predict the relative strengths of ligands for a particular metal ion. However, the Required Spectrochemical series is more specific to a particular metal ion and takes into account the effects of the metal ion’s oxidation state, coordination geometry, and electronic structure on the ligand field strength.
The Required Spectrochemical series is a valuable tool for predicting the properties of metal complexes and designing new ligands with desirable properties for specific applications.
Production of Spectrochemical series
The spectrochemical series is a theoretical concept that is based on experimental measurements of the splitting of d-orbitals in metal ion complexes with different ligands. Therefore, the production of the spectrochemical series involves measuring the splitting of d-orbitals in a set of metal ion complexes with different ligands and then ranking the ligands in order of their ability to cause splitting.
One common experimental method for measuring the splitting of d-orbitals in a metal ion complex is UV-Visible spectroscopy. The spectroscopic properties of the metal ion complexes can be compared to those of a reference compound to determine the degree of splitting.
Another experimental method is Magnetic Circular Dichroism (MCD) spectroscopy, which provides more detailed information about the electronic structure of metal ion complexes and can be used to measure the degree of splitting of the d-orbitals.
Once the experimental data is obtained, the spectrochemical series can be constructed by ranking the ligands in order of their ability to cause splitting of d-orbitals. In practice, the spectrochemical series is often determined for a specific metal ion in a specific coordination geometry and oxidation state.
It is important to note that the spectrochemical series is a theoretical concept and may not fully predict the properties of all metal ion complexes, as other factors such as steric hindrance and solvation effects can also influence the properties of metal complexes.
Case Study on Spectrochemical series
Here is an example case study that illustrates the application of the spectrochemical series in predicting the properties of metal complexes.
Case Study: Designing a Cobalt-based Catalyst for Water Oxidation
Water oxidation is a key reaction in the production of hydrogen as a renewable energy source. One promising approach for water oxidation is to use molecular catalysts based on transition metal complexes. Cobalt-based complexes have shown promising activity for water oxidation, but their properties are highly dependent on the ligands used.
To design a cobalt-based catalyst for water oxidation, the researchers first need to choose a set of ligands to coordinate to the cobalt ion. Based on their knowledge of the spectrochemical series, they decide to choose ligands that are strong field, as these will cause more splitting of the d-orbitals and lead to higher activity.
They choose a set of ligands including tris(2-pyridylmethyl)amine (TPA), 2,2′-bipyridine (bpy), and 1,10-phenanthroline (phen), which are known to be strong field ligands.
Next, they synthesize a series of cobalt complexes with these ligands and measure their activity for water oxidation using electrochemical techniques. They find that the cobalt complexes with TPA and phen ligands show higher activity than those with bpy ligands, consistent with the spectrochemical series prediction.
Furthermore, they use X-ray absorption spectroscopy to probe the electronic structure of the cobalt complexes and find that the TPA and phen ligands cause more splitting of the d-orbitals and lead to a higher number of unpaired electrons in the cobalt ion complex, which is also consistent with the spectrochemical series prediction.
Based on these results, the researchers conclude that the spectrochemical series is a useful tool for predicting the properties of cobalt-based complexes for water oxidation and can be used to guide the design of new ligands for improved activity.
White paper on Spectrochemical series
Here is a white paper on the spectrochemical series, which includes an overview of the concept, its applications in coordination chemistry, and its relevance to various fields of science.
Introduction:
The spectrochemical series is a concept in coordination chemistry that describes the relative strengths of ligands based on their ability to cause splitting of d-orbitals in metal ion complexes. The spectrochemical series is used to predict the properties of metal complexes and to guide the design of new ligands for specific applications.
Overview:
The spectrochemical series is based on the ligand field theory, which describes the interaction between the ligands and the metal ion in a complex. The ligand field theory predicts that the energy levels of the d-orbitals in the metal ion are split into two or more sets of orbitals when ligands are coordinated to the metal ion.
The strength of the ligand field determines the extent of splitting of the d-orbitals and, consequently, the electronic structure and properties of the complex. Ligands that create a strong ligand field cause a large splitting of the d-orbitals and lead to high-spin complexes with unpaired electrons, while ligands that create a weak ligand field cause a small splitting of the d-orbitals and lead to low-spin complexes with paired electrons.
The spectrochemical series is typically represented as a list of ligands arranged in order of their ability to cause splitting of d-orbitals in a metal ion complex, based on a specific metal ion. The order of ligands in the spectrochemical series depends on the properties of the metal ion, including its oxidation state, coordination geometry, and electronic structure.
Applications:
The spectrochemical series is widely used in coordination chemistry and has many applications in various fields of science. Some of the key applications of the spectrochemical series include:
- Design of Metal Complexes: The spectrochemical series is used to predict the properties of metal complexes and to guide the design of new ligands for specific applications. By choosing ligands based on their position in the spectrochemical series, researchers can design metal complexes with desirable properties, such as catalytic activity, magnetic properties, and luminescence.
- Materials Science: The spectrochemical series is used to predict the properties of materials based on their electronic structure. By understanding the electronic structure of materials, researchers can design new materials with specific properties, such as conductivity, hardness, and magnetic properties.
- Medicinal Chemistry: The spectrochemical series is used to design new drugs based on metal complexes. By choosing ligands that create a strong ligand field, researchers can design metal complexes that selectively bind to certain biomolecules and have potential applications in drug delivery and imaging.
- Environmental Chemistry: The spectrochemical series is used to understand the behavior of metal ions in environmental systems. By understanding the electronic structure of metal complexes, researchers can predict the fate and transport of metal ions in natural systems and design methods for remediation of contaminated sites.
Conclusion:
The spectrochemical series is a fundamental concept in coordination chemistry and has many applications in various fields of science. By predicting the properties of metal complexes and guiding the design of new ligands, the spectrochemical series has the potential to impact many areas of research and development.
