Square planar refers to the geometry or arrangement of atoms or ligands around a central atom in a molecule or ion, where the central atom is located at the center of a square plane. In a square planar arrangement, the central atom is surrounded by four ligands or atoms that are arranged in a flat, square configuration with bond angles of 90 degrees.
Square planar geometry is commonly observed in coordination compounds, which are complexes formed by a central metal atom or ion surrounded by ligands. In a square planar complex, the ligands occupy four of the six available coordination sites around the central metal atom, with the other two coordination sites being occupied by other ligands or leaving them unoccupied.
Square planar complexes can have a variety of chemical and physical properties depending on the nature of the central metal atom and the ligands. They are often used in coordination chemistry and have applications in fields such as catalysis, bioinorganic chemistry, and materials science. Some examples of molecules or ions with square planar geometry include tetravalent transition metal complexes such as PtCl4^2-, Ni(CN)4^2-, and Pd(PPh3)2Cl2. Overall, square planar geometry is an important concept in chemistry that describes the spatial arrangement of atoms or ligands around a central atom in certain chemical compounds. So, for example, a molecule with four ligands surrounding a central atom in a square planar arrangement would have a geometry that is described as square planar. This arrangement can affect the chemical and physical properties of the molecule, such as its reactivity, stability, and optical properties. Square planar geometry is an important concept in coordination chemistry, which is the study of complexes formed by a central metal atom or ion surrounded by ligands. Square planar complexes have various applications in fields such as catalysis, bioinorganic chemistry, and materials science. Some examples of molecules or ions with square planar geometry include tetravalent transition metal complexes such as PtCl4^2-, Ni(CN)4^2-, and Pd(PPh3)2Cl2. Overall, square planar geometry is an important concept in chemistry that describes the spatial arrangement of atoms or ligands around a central atom in certain chemical compounds. So, for example, a molecule with four ligands surrounding a central atom in a square planar arrangement would have a geometry that is described as square planar. This arrangement can affect the chemical and physical properties of the molecule, such as its reactivity, stability, and optical properties. Square planar geometry is an important concept in coordination chemistry, which is the study of complexes formed by a central metal atom or ion surrounded by ligands. Square planar complexes have various applications in fields such as catalysis, bioinorganic chemistry, and materials science. Some examples of molecules or ions with square planar geometry include tetravalent transition metal complexes such as PtCl4^2-, Ni(CN)4^2-, and Pd(PPh3)2Cl2. Overall, square planar geometry is an important concept in chemistry that describes the spatial arrangement of atoms or ligands around a central atom in certain chemical compounds. So, for example, a molecule with four ligands surrounding a central atom in a square planar arrangement would have a geometry that is described as square planar. This arrangement can affect the chemical and physical properties of the molecule, such as its reactivity, stability, and optical properties. Square planar geometry is an important concept in coordination chemistry, which is the study of complexes formed by a central metal atom or ion surrounded by ligands.
Square planar molecular geometry

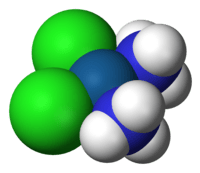
The square planar sub-atomic calculation in science depicts the stereochemistry (spatial course of action of molecules) that is taken on by specific synthetic mixtures. As the name recommends, particles of this math have their iotas situated at the corners.
Splitting of d-orbitals
An overall d-orbital parting graph for square planar (D4h) change metal buildings can be gotten from the general octahedral (Goodness) parting outline, in which the dz2 and the dx2−y2 orbitals are degenerate and higher in energy than the savage arrangement of dxy, dxz and dyz orbitals. At the point when the two hub ligands are taken out to create a square planar math, the dz2 orbital is driven lower in energy as electron repugnance with ligands on the z-pivot is as of now not present. Notwithstanding, for simply σ-giving ligands the dz2 orbital is as yet higher in energy than the dxy, dxz and dyz orbitals due to the torus molded curve of the dz2 orbital. It bears electron thickness on the x-and y-tomahawks and in this manner cooperates with the filled ligand orbitals. The dxy, dxz and dyz orbitals are for the most part introduced as savage however they need to part into two distinct energy levels as for the unchangeable portrayals of the point bunch D4h. Their overall requesting relies upon the idea of the specific complex. Moreover, the parting of d-orbitals is bothered by π-giving ligands rather than octahedral edifices. In the square planar case unequivocally π-giving ligands can cause the dxz and dyz orbitals to be higher in energy than the dz2 orbital, though in the octahedral case π-giving ligands just influence the size of the d-orbital parting and the general requesting of the orbitals is preserved.
When is Required Square planar Chemical Bonding and Molecular Structure
Square planar chemical bonding and molecular structure is required when the central atom in a molecule has a coordination number of four and is surrounded by four ligands arranged in a square planar geometry. This type of molecular arrangement is commonly observed in certain transition metal complexes, where the central metal atom is bonded to four ligands in a square planar configuration.
Square planar geometry is typically observed in transition metal complexes with d^8 or d^10 electron configurations, where the central metal atom has empty or half-filled d orbitals available for bonding. Examples of such complexes include PtCl4^2-, Ni(CN)4^2-, and PdCl2(PPh3)2. In these complexes, the ligands are typically arranged symmetrically around the central metal atom in a square planar arrangement.
The square planar geometry is also observed in some organometallic compounds, particularly those involving metals in the d-block of the periodic table, as well as in some coordination compounds of main group elements. The specific molecular structure and bonding arrangement of a compound are determined by various factors, including the electronic configuration of the central atom, the nature of the ligands, and the steric effects of the ligands, among others.
Square planar geometry has unique properties and reactivity due to the arrangement of the ligands around the central atom. It is important in the field of coordination chemistry and has implications in various chemical reactions and catalytic processes. Understanding the required square planar chemical bonding and molecular structure is crucial in predicting and explaining the properties and behavior of compounds with this geometry. So, square planar chemical bonding and molecular structure are required in specific cases where the central atom has a coordination number of four and is surrounded by four ligands arranged in a square planar configuration.
Where is Required Square planar Chemical Bonding and Molecular Structure
Square planar chemical bonding and molecular structure can be found in various chemical compounds and complexes, particularly those involving transition metals in the d-block of the periodic table. Some examples of where square planar geometry is required or observed include:
- Transition metal complexes: Square planar geometry is commonly observed in certain transition metal complexes, where the central metal atom is bonded to four ligands arranged in a square planar configuration. Examples of such complexes include PtCl4^2-, Ni(CN)4^2-, and PdCl2(PPh3)2.
- Organometallic compounds: Some organometallic compounds involving transition metals can exhibit square planar geometry. These compounds typically contain metal-ligand bonds involving organic ligands, such as cyclopentadienyl (Cp) or phosphine ligands.
- Coordination compounds of main group elements: In some cases, coordination compounds of main group elements, such as Group 14 or Group 16 elements, can also exhibit square planar geometry. For example, certain compounds of tin (Sn) or platinum (Pt) can exhibit square planar molecular structure.
- Catalytic complexes: Square planar complexes can also be involved in catalytic reactions, where the geometry of the complex can influence its catalytic activity. For example, certain transition metal complexes with square planar geometry are used as catalysts in various organic reactions, such as cross-coupling reactions and hydrogenation reactions.
In all of these cases, the square planar geometry is required or observed based on the electronic configuration of the central atom, the nature of the ligands, and other factors that influence the molecular structure and bonding arrangement. Understanding the occurrence and properties of square planar chemical bonding and molecular structure is important in various areas of chemistry, including coordination chemistry, organometallic chemistry, and catalysis.
How is Required Square planar Chemical Bonding and Molecular Structure
Square planar chemical bonding and molecular structure are determined by several factors, including the electronic configuration of the central atom, the nature and arrangement of ligands, and the overall stability and symmetry of the complex.
- Electronic configuration of the central atom: Square planar geometry typically occurs when the central atom has a coordination number of four and the electron configuration allows for the formation of four bonds. Transition metals in the d-block of the periodic table are commonly associated with square planar geometry due to their ability to form multiple coordination bonds with ligands.
- Nature and arrangement of ligands: Ligands are atoms or molecules that form bonds with the central atom in a coordination complex. In square planar complexes, the ligands are typically arranged in a plane around the central atom, forming a square shape. The ligands may be monodentate, meaning they form only one bond with the central atom, or they may be polydentate, forming multiple bonds with the central atom.
- Overall stability and symmetry of the complex: Square planar geometry is often favored in coordination complexes when the ligands are large and bulky, and the resulting complex is more stable in a square planar arrangement. The steric effects of the ligands, as well as the electronic effects, can influence the stability and symmetry of the complex, leading to square planar geometry.
The formation of square planar chemical bonding and molecular structure is typically determined through experimental techniques such as X-ray crystallography, which allows for the determination of the three-dimensional arrangement of atoms in a molecule or complex. Computational methods, such as molecular modeling and quantum chemical calculations, can also be used to predict and understand the geometry of square planar complexes based on the electronic configuration of the central atom and the nature of the ligands.
It’s important to note that the actual geometry of a molecule or complex can also be influenced by other factors such as temperature, solvent effects, and external pressure. The specific requirements for square planar chemical bonding and molecular structure may vary depending on the specific compound or complex under consideration.
Case Study on Square planar Chemical Bonding and Molecular Structure
Sure! Let’s consider a case study on square planar chemical bonding and molecular structure involving a coordination complex.
Case Study: Square Planar Complex of Platinum(II) with Four Ligands
In this case study, we will examine a coordination complex of platinum(II) with four ligands, resulting in a square planar molecular geometry.
- Chemical Species: The central metal atom in this case study is platinum (Pt) with a +2 oxidation state, meaning it has lost two electrons to form a cation. The four ligands that surround the platinum atom are typically monodentate ligands, meaning they form only one bond with the platinum atom. Examples of ligands that could form a square planar complex with platinum(II) include ammonia (NH3), chloride (Cl-), or cyanide (CN-).
- Electronic Configuration: The electronic configuration of platinum(II) is [Xe] 4f14 5d8 6s0, with eight valence electrons available for bonding. The four ligands will donate a total of four electrons to the platinum atom through coordinate covalent bonds, resulting in a total of 12 electrons surrounding the platinum atom.
- Ligand Arrangement: The four ligands will arrange themselves in a square planar arrangement around the platinum atom, forming a flat square shape. The ligands will be positioned in the same plane, with the platinum atom at the center and the ligands at the four corners of the square.
- Stability and Symmetry: The square planar arrangement is favored when the ligands are large and bulky, as it minimizes steric repulsion between the ligands. The resulting complex is stable due to the formation of strong coordinate covalent bonds between the platinum atom and the ligands. The complex will also exhibit high symmetry, with the same ligands arranged symmetrically around the central platinum atom.
- Determination of Geometry: The geometry of the square planar complex can be determined experimentally using techniques such as X-ray crystallography, which allows for the determination of the three-dimensional arrangement of atoms in a crystal lattice. The crystal structure can reveal the positions of the platinum atom and the ligands, confirming the square planar geometry.
- Properties and Applications: Square planar complexes of platinum(II) with four ligands are known to exhibit unique chemical and physical properties, which can be utilized in various applications. For example, platinum-based square planar complexes are commonly used as catalysts in chemical reactions, as they can facilitate the activation of reactants and promote specific reactions. Additionally, platinum-based complexes have been used in medicinal applications, such as in cancer chemotherapy drugs.
In conclusion, the case study of a square planar complex of platinum(II) with four ligands highlights the importance of electronic configuration, ligand arrangement, stability, symmetry, determination of geometry, and properties in understanding square planar chemical bonding and molecular structure. Further research and experimentation in this area can lead to the development of new compounds with unique properties and applications.
White paper on Square planar Chemical Bonding and Molecular Structure
Title: Understanding Square Planar Chemical Bonding and Molecular Structure
Abstract: Square planar chemical bonding and molecular structure are important concepts in coordination chemistry, which involve the arrangement of ligands around a central metal atom in a square planar geometry. This paper aims to provide a comprehensive overview of square planar chemical bonding and molecular structure, including the electronic configuration of metal atoms, ligand arrangement, stability, symmetry, determination of geometry, and properties of square planar complexes. The paper will also discuss the applications of square planar complexes in various fields, such as catalysis and medicinal chemistry. Through a thorough understanding of square planar chemical bonding and molecular structure, researchers and chemists can design and synthesize new compounds with tailored properties for specific applications.
Introduction: Square planar chemical bonding and molecular structure refer to the arrangement of ligands around a central metal atom in a flat square shape, forming a coordination complex. Coordination complexes are widely studied in inorganic chemistry due to their diverse applications in fields such as catalysis, materials science, and medicinal chemistry. Understanding the fundamental principles of square planar chemical bonding and molecular structure is crucial for predicting and manipulating the properties of coordination complexes.
Electronic Configuration of Metal Atoms: The electronic configuration of the central metal atom in a coordination complex plays a key role in determining the geometry of the complex. Transition metal atoms typically form coordination complexes due to their ability to form multiple oxidation states and their availability of d and f orbitals for bonding. The electronic configuration of the metal atom determines the number of valence electrons available for bonding and influences the types of ligands that can coordinate to the metal atom.
Ligand Arrangement: In a square planar complex, the ligands surround the central metal atom in a flat square arrangement. The ligands can be either monodentate or polydentate, forming one or multiple bonds with the metal atom, respectively. The arrangement of the ligands is influenced by various factors, including steric repulsion, electronic properties of the ligands, and coordination number of the metal atom.
Stability and Symmetry: The stability of a square planar complex depends on the strength of the coordinate covalent bonds formed between the metal atom and the ligands. Ligands that are large and bulky tend to favor a square planar arrangement, as it minimizes steric repulsion. The symmetry of the square planar complex is also important, as it can affect the properties and reactivity of the complex. Square planar complexes typically exhibit high symmetry, with the ligands arranged symmetrically around the central metal atom.
Determination of Geometry: The determination of the geometry of a square planar complex can be done experimentally using techniques such as X-ray crystallography, which allows for the determination of the three-dimensional arrangement of atoms in a crystal lattice. Other spectroscopic methods, such as NMR, UV-Vis, and IR, can also provide information about the geometry and electronic properties of square planar complexes.
Properties and Applications: Square planar complexes exhibit unique properties that can be utilized in various applications. For example, square planar complexes of transition metals are commonly used as catalysts in chemical reactions due to their ability to activate reactants and promote specific reactions. Additionally, square planar complexes have been used in medicinal applications, such as in cancer chemotherapy drugs. The properties and reactivity of square planar complexes can be tailored by modifying the ligands, metal atom, and other factors, providing opportunities for designing new compounds with desired properties.
Conclusion:
In conclusion, square planar chemical bonding and molecular structure play a significant role in coordination chemistry, offering insights into the arrangement of ligands around a central metal atom in a flat square shape. The stability and symmetry of square planar complexes are influenced by factors such as steric repulsion, electronic properties of ligands, and coordination number of the metal atom. Experimental techniques such as X-ray crystallography and spectroscopic methods can be employed to determine the geometry and properties of square planar complexes. The unique properties of square planar complexes, such as their catalytic activity and medicinal applications, make them valuable in various fields of research and applications. Further studies and research in square planar chemical bonding and molecular structure will continue to advance our understanding of coordination chemistry and contribute to the development of new compounds with tailored properties for specific applications. Overall, square planar chemical bonding and molecular structure are important topics in inorganic chemistry, offering immense potential for advancements in diverse areas of science and technology.
