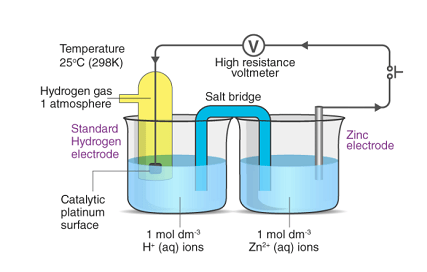
Standard electrode potentials (also known as standard reduction potentials) are a measure of the tendency of a chemical species to undergo reduction or oxidation under standard conditions. These potentials are reported relative to the standard hydrogen electrode (SHE), which is assigned a potential of 0.00 volts.
Standard electrode potentials are typically represented using the notation E°, where the superscript “°” indicates standard conditions (i.e., 1 atm pressure, 25°C temperature, and 1 M concentration of the relevant species). For example, the standard electrode potential for the reduction of Cu2+ to Cu is +0.34 V, which means that under standard conditions, Cu2+ ions have a greater tendency to gain electrons and be reduced to Cu than hydrogen ions do.
The sign of the standard electrode potential indicates the direction of electron flow: a positive value indicates that the species is reduced (gains electrons), while a negative value indicates that the species is oxidized (loses electrons). Standard electrode potentials are important in electrochemistry, as they allow us to predict the direction of spontaneous electron transfer reactions and to calculate the voltage of electrochemical cells.
What is Required Standard electrode potentials
“Required standard electrode potentials” is not a commonly used term in chemistry, and I am not sure what you are referring to.
However, I can provide some information about standard electrode potentials in general. As I mentioned earlier, standard electrode potentials (also called standard reduction potentials) are a measure of the tendency of a chemical species to undergo reduction or oxidation under standard conditions. These potentials are important in electrochemistry because they allow us to predict the direction of spontaneous electron transfer reactions and to calculate the voltage of electrochemical cells.
To determine the standard electrode potential of a particular species, a half-cell containing the species is connected to a standard hydrogen electrode (SHE) in a galvanic cell, and the potential difference between the two half-cells is measured. The measured potential difference is equal to the difference in standard electrode potentials between the two half-cells.
In some cases, it may be necessary to determine the standard electrode potential of a species experimentally because it is not known or cannot be calculated from other data. In other cases, the standard electrode potential may be provided in a reference table, such as the CRC Handbook of Chemistry and Physics.
When is Required Standard electrode potentials
Standard electrode potentials are used in a variety of contexts in chemistry, particularly in electrochemistry. They are important for predicting the direction of electron transfer in redox reactions and for calculating the voltage of electrochemical cells.
In practical terms, standard electrode potentials are required whenever we need to determine the relative strengths of oxidizing and reducing agents and their ability to participate in redox reactions. For example, standard electrode potentials can be used to predict whether a particular metal will undergo corrosion in a given environment or to design and optimize electrochemical processes such as batteries, fuel cells, and electrolysis cells.
Standard electrode potentials are also required for the construction of Pourbaix diagrams, which show the stability of different species in aqueous solutions as a function of pH and potential. Pourbaix diagrams are used to study corrosion and passivation behavior of metals and alloys, and to design and optimize electrochemical systems for various applications.
Overall, standard electrode potentials are a fundamental concept in electrochemistry and are required whenever we need to understand or manipulate electron transfer reactions in aqueous or non-aqueous environments.
Where is Required Standard electrode potentials
Standard electrode potentials are a concept in chemistry and are not physically located in any specific place. They are a fundamental part of the discipline of electrochemistry and are used by scientists and engineers around the world in a wide range of applications.
Standard electrode potentials are typically referenced in scientific literature, textbooks, and online resources such as databases, calculators, and simulation tools. They are also used in laboratory settings to design and optimize electrochemical experiments and processes.
Some common sources of standard electrode potential data include the CRC Handbook of Chemistry and Physics, the NIST Chemistry WebBook, and online databases such as the Electrochemical Data and Technology Portal. However, it is important to note that the values of standard electrode potentials can vary depending on the conditions (e.g., temperature, pressure, concentration) under which they are measured, and that they should always be used in the appropriate context and with proper consideration of potential uncertainties and limitations.
How is Required Standard electrode potentials
Standard electrode potentials (also called standard reduction potentials) are typically measured using a half-cell reaction and a reference electrode, such as the standard hydrogen electrode (SHE). In general, a half-cell reaction involves the transfer of electrons from one chemical species (the oxidizing agent) to another chemical species (the reducing agent). By measuring the potential difference between the half-cell reaction and the reference electrode, the standard electrode potential can be determined.
The standard electrode potential is defined as the potential difference between the half-cell reaction and the SHE, both at standard conditions (i.e., 25°C, 1 atm pressure, and 1 M concentration of the relevant species). The standard electrode potential is measured in volts (V) and is often denoted by the symbol E°.
To measure the standard electrode potential of a given chemical species, a half-cell is constructed using the species of interest and the reference electrode, and the potential difference between the two electrodes is measured using a voltmeter. The measured potential difference is equal to the difference in standard electrode potentials between the two electrodes.
The standard electrode potential can also be calculated using thermodynamic data, such as the Gibbs free energy of formation of the species involved in the half-cell reaction. The Nernst equation is often used to relate the standard electrode potential to the concentrations of the relevant species and the temperature of the system.
Overall, standard electrode potentials are determined experimentally or calculated theoretically, and are essential for understanding and predicting the behavior of electrochemical systems.
Production of Standard electrode potentials
Standard electrode potentials are not “produced” in the same sense that a physical object is produced. Rather, they are determined experimentally or calculated theoretically based on the properties of the chemical species involved in the redox reaction.
Experimental determination of standard electrode potentials involves measuring the potential difference between the half-cell reaction and a reference electrode, such as the standard hydrogen electrode (SHE), under standard conditions of temperature, pressure, and concentration. The potential difference measured in this way is equal to the difference in standard electrode potentials between the half-cell reaction and the SHE.
The standard electrode potentials can also be calculated theoretically using thermodynamic data, such as the Gibbs free energy of formation of the chemical species involved in the redox reaction. The Nernst equation is often used to relate the standard electrode potential to the concentrations of the relevant species and the temperature of the system.
Once the standard electrode potentials are determined or calculated, they can be used to predict the direction and extent of redox reactions, to calculate the voltage of electrochemical cells, and to design and optimize electrochemical processes.
In summary, standard electrode potentials are not produced but rather determined experimentally or calculated theoretically based on the properties of the chemical species involved in the redox reaction.
Case Study on Standard electrode potentials
One example of the practical application of standard electrode potentials is in the design and optimization of electrochemical cells, such as batteries or fuel cells. These devices rely on redox reactions to generate or store electrical energy, and their performance is strongly influenced by the standard electrode potentials of the chemical species involved.
For instance, consider the case of a lithium-ion battery, which is a type of rechargeable battery commonly used in portable electronic devices. The battery consists of two electrodes, a lithium cobalt oxide (LiCoO2) cathode and a graphite anode, separated by an electrolyte solution. During charging, lithium ions are extracted from the cathode and inserted into the anode, and the reverse process occurs during discharging, generating an electric current.
The standard electrode potentials of the cathode and anode materials play a crucial role in the performance of the battery. The cathode has a high standard electrode potential (+3.0 V vs SHE), which means it has a strong tendency to accept electrons and become reduced. The anode has a lower standard electrode potential (-0.3 V vs SHE), which means it has a strong tendency to donate electrons and become oxidized.
By exploiting the difference in standard electrode potentials between the cathode and anode, the lithium-ion battery can efficiently generate and store electrical energy. During charging, an external power source provides electrons to the cathode, reducing it to its lower-energy state and allowing lithium ions to be extracted. At the same time, the anode releases electrons, which are stored in the external circuit as electrical energy. During discharging, the reverse process occurs, with the anode accepting electrons from the external circuit, reducing the lithium ions and generating electrical energy.
The specific design of a lithium-ion battery, including the choice of cathode and anode materials, the electrolyte composition, and the cell geometry, is determined based on a variety of factors, including the desired energy density, power density, cycle life, and safety. The standard electrode potentials of the materials used play a critical role in determining the overall performance and efficiency of the battery.
Overall, this case study demonstrates how an understanding of standard electrode potentials can be used to design and optimize electrochemical cells, such as batteries or fuel cells, for a wide range of applications.
White paper on Standard electrode potentials
Introduction:
Electrochemical reactions play a vital role in many areas of chemistry, physics, and materials science. They involve the transfer of electrons from one chemical species to another, resulting in changes in the oxidation states of the reactants. The tendency of a chemical species to gain or lose electrons is quantified by its standard electrode potential, which is a fundamental thermodynamic property that can be used to predict the direction and extent of redox reactions. This white paper aims to provide an overview of standard electrode potentials, including their definition, measurement, and application.
Definition:
The standard electrode potential is defined as the potential difference between the half-cell reaction and the standard hydrogen electrode (SHE), both at standard conditions of temperature, pressure, and concentration. The SHE is a reference electrode with a fixed potential of 0.0 V vs SHE, which serves as a standard against which other electrode potentials are measured. The standard electrode potential is measured in volts (V) and is often denoted by the symbol E°.
Measurement:
Experimental determination of standard electrode potentials involves measuring the potential difference between the half-cell reaction and the SHE, under standard conditions of temperature, pressure, and concentration. A half-cell reaction involves the transfer of electrons from one chemical species (the oxidizing agent) to another chemical species (the reducing agent). By measuring the potential difference between the half-cell reaction and the SHE, the standard electrode potential can be determined. For example, the standard electrode potential of the reduction of copper ions to copper metal can be determined by setting up a half-cell reaction with a copper electrode and a solution of copper ions, and measuring the potential difference between the copper electrode and the SHE.
Calculation:
The standard electrode potential can also be calculated theoretically using thermodynamic data, such as the Gibbs free energy of formation of the chemical species involved in the redox reaction. The Nernst equation is often used to relate the standard electrode potential to the concentrations of the relevant species and the temperature of the system. For example, the standard electrode potential of the reduction of ferricyanide ions to ferrocyanide ions can be calculated using the Nernst equation, given the concentrations of the species involved.
Application:
Standard electrode potentials are essential for understanding and predicting the behavior of electrochemical systems, including batteries, fuel cells, and corrosion processes. The specific design of electrochemical cells, including the choice of electrode materials, the electrolyte composition, and the cell geometry, is determined based on a variety of factors, including the desired energy density, power density, cycle life, and safety. The standard electrode potentials of the materials used play a critical role in determining the overall performance and efficiency of the cell. For example, the choice of cathode and anode materials in a lithium-ion battery is determined based on their standard electrode potentials, which influence the energy density and cycle life of the battery.
Conclusion:
Standard electrode potentials are a fundamental thermodynamic property that can be used to predict the direction and extent of redox reactions. They are determined experimentally or calculated theoretically based on the properties of the chemical species involved in the redox reaction. Standard electrode potentials are essential for understanding and predicting the behavior of electrochemical systems, including batteries, fuel cells, and corrosion processes, and their application is critical for designing and optimizing these systems for a wide range of applications.
