Structural isomerism and geometrical isomerism are two different types of isomerism in organic chemistry.
Structural isomerism occurs when molecules have the same molecular formula but different arrangements of atoms. This can be due to differences in the bonding patterns of the atoms within the molecule. For example, pentane and 2-methylbutane have the same molecular formula (C5H12) but different structures, making them structural isomers.
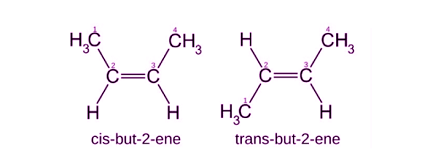
Geometrical isomerism, also known as cis-trans isomerism, occurs when molecules have the same molecular formula and the same bonding pattern, but different spatial arrangements of atoms due to the presence of double bonds or rings in the molecule. In this type of isomerism, the atoms are connected in the same order, but their arrangement in space is different due to restricted rotation around the double bond or ring. For example, cis-2-butene and trans-2-butene have the same molecular formula (C4H8) and the same bonding pattern, but different spatial arrangements of atoms around the double bond, making them geometrical isomers.
In summary, structural isomerism refers to differences in the arrangement of atoms within a molecule, while geometrical isomerism refers to differences in the spatial arrangement of atoms due to the presence of double bonds or rings in the molecule.
What is Required Basic Principles of Organic Chemistry Structural and Geometrical isomerism
The basic principles of organic chemistry that are required to understand structural and geometrical isomerism include:
- Molecular formula: The molecular formula of a compound provides information about the types and numbers of atoms present in the molecule.
- Structural formula: The structural formula of a compound provides information about the bonding patterns between the atoms in the molecule.
- Bonding theory: Understanding the principles of covalent bonding is crucial to understanding the structural isomerism, as the bonding pattern is what distinguishes one isomer from another.
- Stereochemistry: Stereochemistry is the study of the three-dimensional arrangements of atoms in molecules. It is essential to understanding geometrical isomerism, which arises due to the different arrangements of atoms in space.
- Isomerism: Isomerism is the existence of two or more compounds with the same molecular formula but different structural or spatial arrangements. Understanding the concept of isomerism is crucial in identifying and distinguishing between different types of isomers, including structural and geometrical isomers.
- Nomenclature: A systematic and standardized naming system is essential in organic chemistry to communicate chemical information effectively. Knowledge of nomenclature rules is necessary to name and classify different isomers.
Overall, a fundamental understanding of these principles is necessary to understand structural and geometrical isomerism in organic chemistry.
Who is Required Basic Principles of Organic Chemistry Structural and Geometrical isomerism
The required basic principles of organic chemistry for understanding structural and geometrical isomerism are important for anyone studying or working in the field of organic chemistry. This includes:
- Students: Students studying organic chemistry at the high school, college, or graduate level need to understand the basic principles of organic chemistry, including bonding theory, stereochemistry, isomerism, and nomenclature, to comprehend the concepts of structural and geometrical isomerism.
- Chemists: Chemists working in the field of organic chemistry, such as medicinal chemists, synthetic chemists, or analytical chemists, need a thorough understanding of the basic principles of organic chemistry, including structural and geometrical isomerism, to design, synthesize, and analyze organic molecules.
- Researchers: Researchers involved in drug discovery, materials science, or chemical engineering, for example, need to understand the principles of organic chemistry to identify, isolate, and study different isomers and their properties.
In summary, anyone involved in the field of organic chemistry, including students, chemists, and researchers, requires a solid understanding of the basic principles of organic chemistry to comprehend the concepts of structural and geometrical isomerism.
When is Required Basic Principles of Organic Chemistry Structural and Geometrical isomerism
The required basic principles of organic chemistry for understanding structural and geometrical isomerism are necessary in various situations, including:
- Organic chemistry coursework: Students studying organic chemistry at the high school, college, or graduate level require a strong foundation in the basic principles of organic chemistry, including isomerism, to comprehend the course material and succeed in their coursework.
- Synthesis of organic molecules: Chemists involved in synthesizing organic molecules need to understand the basic principles of organic chemistry, including bonding theory, stereochemistry, isomerism, and nomenclature, to design and synthesize specific isomers and avoid unwanted isomers.
- Identification and analysis of organic molecules: Researchers involved in the identification and analysis of organic molecules, such as those in drug discovery or materials science, need to understand the basic principles of organic chemistry to identify and distinguish between different isomers and their properties.
- Interpretation of spectroscopic data: Analytical chemists and researchers involved in spectroscopic analysis of organic molecules, such as NMR, IR, or UV-Vis spectroscopy, need to understand the basic principles of organic chemistry, including isomerism, to interpret and assign spectroscopic data to specific isomers.
In summary, a strong understanding of the basic principles of organic chemistry, including isomerism, is required in various situations, including organic chemistry coursework, synthesis of organic molecules, identification and analysis of organic molecules, and interpretation of spectroscopic data.
Where is Required Basic Principles of Organic Chemistry Structural and Geometrical isomerism
The required basic principles of organic chemistry for understanding structural and geometrical isomerism are necessary in various fields and settings, including:
- Academic institutions: Organic chemistry is a fundamental course in many academic institutions, including high schools, colleges, and universities. The basic principles of organic chemistry, including isomerism, are taught in these institutions to provide students with a strong foundation in organic chemistry.
- Research laboratories: Research laboratories involved in organic chemistry research, such as those focused on drug discovery, materials science, or chemical engineering, require a strong understanding of the basic principles of organic chemistry, including isomerism, to design and synthesize specific isomers and analyze their properties.
- Industrial settings: The principles of organic chemistry, including isomerism, are essential in various industries, such as pharmaceuticals, agrochemicals, and materials science. Industrial chemists require a strong foundation in the basic principles of organic chemistry to design and synthesize specific isomers and analyze their properties.
- Government agencies: Government agencies involved in regulating industries that use organic chemistry, such as the Food and Drug Administration (FDA), require a strong understanding of the basic principles of organic chemistry, including isomerism, to evaluate and approve new drugs and other organic chemicals.
In summary, the required basic principles of organic chemistry for understanding structural and geometrical isomerism are necessary in various fields and settings, including academic institutions, research laboratories, industrial settings, and government agencies.
How is Required Basic Principles of Organic Chemistry Structural and Geometrical isomerism
The required basic principles of organic chemistry for understanding structural and geometrical isomerism can be learned through various methods, including:
- Textbooks and lectures: Organic chemistry textbooks and lectures provide a comprehensive introduction to the basic principles of organic chemistry, including isomerism. Students can learn the principles of isomerism through the study of concepts such as bonding theory, stereochemistry, and nomenclature.
- Laboratory experiments: Laboratory experiments provide hands-on experience in organic chemistry, allowing students to apply the basic principles of organic chemistry, including isomerism, in practice. Laboratory experiments can also provide opportunities to synthesize and analyze isomers.
- Online resources: There are numerous online resources available for learning the basic principles of organic chemistry, including isomerism. These resources may include video lectures, interactive tutorials, and practice problems.
- Professional development courses: Professional development courses, workshops, and seminars can provide opportunities for chemists to improve their understanding of the basic principles of organic chemistry, including isomerism. These courses can be found through academic institutions, professional organizations, and industry associations.
In summary, the required basic principles of organic chemistry for understanding structural and geometrical isomerism can be learned through a variety of methods, including textbooks and lectures, laboratory experiments, online resources, and professional development courses.
Case Study on Basic Principles of Organic Chemistry Structural and Geometrical isomerism
Case Study: Identifying Isomers in Drug Design
The basic principles of organic chemistry, including structural and geometrical isomerism, play a critical role in drug design. Isomers can have different biological activities and pharmacological properties, making it essential for chemists to identify and distinguish between different isomers.
Consider the following case study of identifying isomers in drug design:
A pharmaceutical company is developing a new drug to treat a specific disease. The lead compound for the drug has been synthesized and shows promising activity in early testing. However, the lead compound has multiple isomers, which can have different pharmacological properties.
The chemists at the pharmaceutical company need to identify and distinguish between different isomers to determine which isomer is most active against the disease target and has the best drug-like properties. They use the basic principles of organic chemistry, including isomerism, to identify and characterize the different isomers.
First, they use spectroscopic techniques, such as NMR and IR, to determine the connectivity and functional groups present in the lead compound and its isomers. They observe differences in the spectra of the isomers, indicating that they have different structures.
Next, they use molecular modeling software to generate three-dimensional structures of the different isomers. They observe that some isomers have different arrangements of substituents around double bonds, indicating that they are geometrical isomers. Other isomers have different arrangements of substituents around the carbon backbone, indicating that they are structural isomers.
Finally, they synthesize and test each isomer to determine its biological activity and pharmacological properties. They observe that some isomers have much higher activity against the disease target and better drug-like properties than others, allowing them to select the best isomer for further development.
In conclusion, the basic principles of organic chemistry, including isomerism, play a critical role in drug design. Chemists can use spectroscopic techniques, molecular modeling, and synthesis and testing to identify and distinguish between different isomers, allowing them to select the most active and drug-like isomer for further development.
White paper on Basic Principles of Organic Chemistry Structural and Geometrical isomerism
Introduction:
Organic chemistry is the study of carbon-containing compounds and their properties, reactions, and synthesis. One of the fundamental concepts in organic chemistry is isomerism, which refers to the existence of compounds with the same molecular formula but different structures. Structural isomers have different arrangements of atoms in their molecules, while geometrical isomers have the same arrangement of atoms but different spatial arrangements. Understanding isomerism is critical for organic chemists, as it can have significant implications for the properties and behavior of organic compounds.
Basic Principles of Structural Isomerism:
Structural isomerism arises from differences in the arrangement of atoms within a molecule. The basic principles of structural isomerism include:
- Chain Isomerism: Chain isomers have the same molecular formula but differ in the arrangement of the carbon chain. For example, pentane and 2-methylbutane have the same molecular formula (C5H12) but different arrangements of the carbon chain.
- Positional Isomerism: Positional isomers have the same molecular formula but differ in the position of functional groups or substituents on the carbon chain. For example, propanol and 2-propanol have the same molecular formula (C3H8O) but differ in the position of the hydroxyl group.
- Functional Group Isomerism: Functional group isomers have the same molecular formula but differ in the functional group present in the molecule. For example, ethanol and dimethyl ether have the same molecular formula (C2H6O) but differ in the functional group present in the molecule.
Basic Principles of Geometrical Isomerism:
Geometrical isomerism arises from differences in the spatial arrangement of atoms within a molecule. The basic principles of geometrical isomerism include:
- Cis-Trans Isomerism: Cis-trans isomers have the same molecular formula and the same connectivity of atoms but differ in the spatial arrangement of substituents around a double bond. Cis isomers have substituents on the same side of the double bond, while trans isomers have substituents on opposite sides of the double bond. For example, cis-2-butene and trans-2-butene have the same molecular formula (C4H8) and the same connectivity of atoms but differ in the spatial arrangement of substituents around the double bond.
- Optical Isomerism: Optical isomers, also known as enantiomers, have the same molecular formula and the same connectivity of atoms but differ in the spatial arrangement of atoms around a chiral center. Chiral centers are carbon atoms with four different substituents attached to them. Optical isomers are non-superimposable mirror images of each other and have different optical properties. For example, D-glucose and L-glucose are optical isomers that have the same molecular formula (C6H12O6) and the same connectivity of atoms but differ in the spatial arrangement of atoms around their chiral centers.
Importance of Understanding Isomerism:
Understanding isomerism is critical for organic chemists as it can have significant implications for the properties and behavior of organic compounds. For example, different isomers of a compound can have different physical properties such as boiling points, melting points, and solubilities, which can affect their separation and purification. Isomerism can also affect the biological activity and pharmacological properties of organic compounds, making it essential for drug design.
Conclusion:
In conclusion, the basic principles of organic chemistry structural and geometrical isomerism are essential concepts for understanding the properties and behavior of organic compounds. Structural isomers differ in the arrangement of atoms within a molecule, while geometrical isomers differ in the spatial arrangement of atoms. Understanding isomerism is critical for organic chemists as it can affect the physical, chemical, and biological properties of compounds, making it essential for drug design and other applications. By studying isomerism, organic chemists can gain insights into the structure and reactivity of organic compounds, enabling them to design new molecules with specific properties and applications. Overall, isomerism is a fundamental concept that underpins much of organic chemistry and is essential for anyone studying or working in this field.
