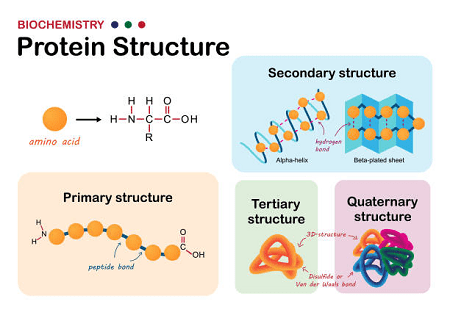
Peptides are short chains of amino acids that are linked together by peptide bonds. The structure of a peptide can be described at several levels: primary, secondary, tertiary, and quaternary.
- Primary structure: The primary structure of a peptide refers to the linear sequence of amino acids in the peptide chain. The sequence of amino acids is determined by the genetic code, and it is critical for determining the peptide’s properties and functions.
- Secondary structure: The secondary structure of a peptide refers to the local folding or bending of the peptide chain. The most common types of secondary structures are alpha helices and beta sheets. Alpha helices are formed when the peptide chain twists into a spiral, while beta sheets are formed when the peptide chain folds back and forth like an accordion. The formation of these secondary structures is stabilized by hydrogen bonding between the amino acid residues.
Overall, the secondary structure is determined by the primary sequence of amino acids, as well as by the environment of the peptide (e.g., temperature, pH, solvent).
- Tertiary structure: The tertiary structure of a peptide refers to the three-dimensional arrangement of the peptide chain. The tertiary structure is determined by a combination of factors, including the primary and secondary structures, as well as the interactions between the side chains of the amino acid residues. These interactions include hydrogen bonding, electrostatic interactions, and van der Waals forces.
- Quaternary structure: The quaternary structure of a peptide refers to the arrangement of multiple peptide chains in a larger protein complex. The individual peptide chains in the complex may have their own primary, secondary, and tertiary structures, but the quaternary structure describes how they come together to form a larger functional unit.
In summary, the primary structure of a peptide is the linear sequence of amino acids, while the secondary structure refers to local folding or bending of the peptide chain. The tertiary structure is the overall three-dimensional arrangement of the peptide chain, and the quaternary structure describes how multiple peptide chains come together to form a larger functional unit.
What is Required Structure of peptides (Primary and Secondary)
The required structure of peptides depends on their intended function. However, there are some general principles that apply to the primary and secondary structures of most peptides.
- Primary structure: The primary structure of a peptide is determined by the sequence of amino acids in the peptide chain. This sequence is critical for determining the peptide’s properties and functions, as it determines how the peptide will fold into its final three-dimensional structure. The correct primary structure is necessary for the peptide to perform its intended function.
- Secondary structure: The secondary structure of a peptide is determined by the local folding or bending of the peptide chain. The most common types of secondary structures are alpha helices and beta sheets. These secondary structures are stabilized by hydrogen bonding between the amino acid residues.
The required secondary structure of a peptide will depend on its function. For example, a peptide that needs to bind to a specific protein may require a specific secondary structure that complements the binding site on the protein. Alternatively, a peptide that needs to be flexible and adopt different conformations may require a more flexible secondary structure.
In summary, the required structure of peptides depends on their intended function. The primary structure determines the sequence of amino acids in the peptide chain, while the secondary structure determines the local folding or bending of the peptide chain. The correct primary and secondary structures are necessary for the peptide to perform its intended function.
When is Required Structure of peptides (Primary and Secondary)
The required structure of peptides is important in many fields, including biochemistry, biotechnology, pharmacology, and medicine. Here are some specific examples of when the required structure of peptides is particularly important:
- Drug design: Peptides can be used as drugs to treat a variety of diseases, including cancer, diabetes, and autoimmune disorders. In drug design, it is critical to determine the required primary and secondary structures of the peptide to ensure that it can effectively bind to its target protein and carry out its intended function.
- Protein engineering: Peptides can also be used to engineer new proteins with specific functions. In protein engineering, the required primary and secondary structures of the peptide can be used as a starting point to design new protein sequences that have desired properties.
- Biomaterials: Peptides can be used to create biomaterials for a variety of applications, such as tissue engineering and drug delivery. In biomaterials design, the required primary and secondary structures of the peptide can be used to optimize the properties of the material, such as its strength, stability, and biocompatibility.
- Molecular biology: Peptides play important roles in many biological processes, such as DNA replication, transcription, and translation. In molecular biology, it is important to understand the required primary and secondary structures of peptides involved in these processes to understand their functions and mechanisms.
In summary, the required structure of peptides is important in a wide range of applications, including drug design, protein engineering, biomaterials, and molecular biology. Understanding the required primary and secondary structures of peptides is critical to ensuring that they can effectively perform their intended functions.
Where is Required Structure of peptides (Primary and Secondary)
The required structure of peptides (primary and secondary) can be found or determined in several ways, depending on the application and the specific peptide of interest. Here are some examples:
- Protein crystallography: Protein crystallography is a technique that can be used to determine the three-dimensional structure of proteins, including peptides. By analyzing the diffraction pattern of X-rays passing through a crystal of the protein, the positions of the atoms in the protein can be determined. This technique is particularly useful for determining the tertiary structure of peptides.
- Nuclear magnetic resonance (NMR) spectroscopy: NMR spectroscopy is a technique that can be used to determine the three-dimensional structure of proteins, including peptides, in solution. By analyzing the interaction of the protein with a magnetic field, the positions of the atoms in the protein can be determined. This technique is particularly useful for determining the secondary structure of peptides.
- Computational methods: Computational methods, such as molecular dynamics simulations and homology modeling, can be used to predict the structure of peptides based on their amino acid sequence. These methods rely on algorithms and models that simulate the behavior of molecules and atoms, and they can provide valuable insights into the structure and function of peptides.
- Experimental methods: Experimental methods, such as circular dichroism (CD) spectroscopy, can be used to determine the secondary structure of peptides in solution. CD spectroscopy measures the difference in absorbance of left- and right-circularly polarized light, which is sensitive to the secondary structure of the peptide.
In summary, the required structure of peptides (primary and secondary) can be found or determined through a variety of techniques, including protein crystallography, NMR spectroscopy, computational methods, and experimental methods such as CD spectroscopy. The choice of technique will depend on the specific peptide of interest and the application.
How is Required Structure of peptides (Primary and Secondary)
The required structure of peptides (primary and secondary) can be determined through a variety of experimental and computational methods. Here are some common methods used to determine the structure of peptides:
- X-ray crystallography: X-ray crystallography is a powerful technique used to determine the three-dimensional structure of proteins, including peptides. In this method, a crystal of the protein is formed, and X-rays are directed at the crystal. The X-rays are diffracted by the atoms in the protein, and the resulting diffraction pattern is used to reconstruct the three-dimensional structure of the protein.
- Nuclear Magnetic Resonance (NMR) spectroscopy: NMR spectroscopy is a technique used to study the structure and dynamics of molecules, including peptides, in solution. In this method, the peptide is dissolved in a suitable solvent, and a magnetic field is applied. The resulting magnetic properties of the peptide are measured, which can be used to determine the structure of the peptide.
- Circular Dichroism (CD) spectroscopy: CD spectroscopy is a technique used to study the secondary structure of peptides. In this method, polarized light is passed through the peptide, and the difference in the absorption of left- and right-circularly polarized light is measured. This difference is sensitive to the secondary structure of the peptide, and can be used to determine whether it is in an alpha-helix, beta-sheet, or random coil conformation.
- Computational methods: Computational methods, such as molecular dynamics simulations and homology modeling, can be used to predict the structure of peptides based on their amino acid sequence. These methods rely on algorithms and models that simulate the behavior of molecules and atoms, and can provide valuable insights into the structure and function of peptides.
In summary, the required structure of peptides (primary and secondary) can be determined through a variety of experimental and computational methods. The choice of method will depend on the specific peptide of interest and the application.
Production of Structure of peptides (Primary and Secondary)
The production of peptides with a specific structure (primary and secondary) can be achieved through several methods, including chemical synthesis and recombinant DNA technology.
- Chemical Synthesis: Chemical synthesis involves the stepwise assembly of amino acids to form a peptide chain. This approach is commonly used to produce small peptides and can be used to introduce specific modifications or labels to the peptide. The chemical synthesis of peptides can be performed manually or using automated peptide synthesizers. The final product can be purified using techniques such as high-performance liquid chromatography (HPLC) or flash chromatography.
- Recombinant DNA technology: Recombinant DNA technology involves the use of genetically modified organisms, such as bacteria or yeast, to produce peptides with a specific structure. In this method, the gene encoding the peptide is inserted into a plasmid vector and then introduced into the host organism. The host organism then produces the peptide through transcription and translation of the inserted gene. The peptide can be purified from the host organism using techniques such as ion exchange chromatography or affinity chromatography.
- Chemical modification of natural peptides: Natural peptides can also be chemically modified to produce peptides with a specific structure. Chemical modification involves altering the structure of the peptide by introducing specific chemical groups or modifying the amino acid residues. This approach can be used to introduce specific structural features such as disulfide bonds, which can stabilize the secondary structure of the peptide.
In summary, the production of peptides with a specific structure (primary and secondary) can be achieved through chemical synthesis, recombinant DNA technology, or chemical modification of natural peptides. The choice of method will depend on the specific peptide of interest, the desired structure, and the intended application.
Case Study on Structure of peptides (Primary and Secondary)
One example of the importance of understanding the structure of peptides is the development of peptide-based therapeutics.
One such case study is the development of GLP-1 agonists for the treatment of type 2 diabetes. GLP-1 is a hormone that stimulates insulin secretion and reduces blood glucose levels. However, GLP-1 is rapidly degraded in the body by the enzyme dipeptidyl peptidase-4 (DPP-4), limiting its therapeutic potential.
To overcome this limitation, researchers developed GLP-1 agonists, which are peptides that mimic the action of GLP-1 but are resistant to degradation by DPP-4. The structure of GLP-1 agonists is critical to their function, as it determines their ability to bind to and activate the GLP-1 receptor.
The primary structure of GLP-1 agonists is typically composed of 30-40 amino acids, with specific modifications to enhance their stability and activity. For example, Exenatide, a GLP-1 agonist, is a synthetic peptide that contains a modified version of GLP-1 with a C-terminal extension that enhances its stability and duration of action.
The secondary structure of GLP-1 agonists is also important, as it influences their ability to interact with the GLP-1 receptor. GLP-1 agonists typically adopt an alpha-helical conformation, which is stabilized by intramolecular disulfide bonds. This conformation allows them to bind to the GLP-1 receptor with high affinity and specificity, leading to increased insulin secretion and glucose uptake.
The development of GLP-1 agonists highlights the importance of understanding the structure of peptides, particularly for the development of peptide-based therapeutics. The structure of peptides influences their stability, activity, and specificity, and can be optimized to enhance their therapeutic potential.
White paper on Structure of peptides (Primary and Secondary)
Here is a white paper on the structure of peptides, with a focus on their primary and secondary structure, their importance, and their applications in various fields.
Introduction:
Peptides are short chains of amino acids that are essential building blocks of proteins. They play critical roles in a wide range of biological processes, including hormone signaling, enzyme activity, and immune response. The structure of peptides is important for their function, as it determines their stability, activity, and specificity. Peptides can adopt different structures, including primary, secondary, tertiary, and quaternary structures. This white paper will focus on the primary and secondary structure of peptides, their importance, and their applications in various fields.
Primary Structure of Peptides:
The primary structure of a peptide is its sequence of amino acids. Peptides are composed of 20 different amino acids, each with a unique side chain. The order and composition of amino acids in a peptide determine its primary structure. The primary structure of a peptide is critical for its stability and activity. Any changes or modifications to the primary structure can significantly alter the peptide’s properties.
Secondary Structure of Peptides:
The secondary structure of a peptide refers to the arrangement of amino acids in a peptide chain. Peptides can adopt different secondary structures, including alpha-helices, beta-sheets, and random coils. The secondary structure of a peptide is stabilized by hydrogen bonds between the backbone atoms of amino acids. The secondary structure of a peptide is important for its stability, activity, and specificity. It determines the peptide’s ability to interact with other molecules and perform its biological function.
Importance of the Structure of Peptides:
The structure of peptides is critical for their function and applications in various fields. Peptides with specific structures can be used as therapeutics, diagnostic tools, and research reagents. For example, peptide hormones such as insulin and glucagon are used to treat diabetes. Peptide antibiotics such as penicillin are used to treat bacterial infections. Peptides with specific structures can also be used as molecular probes to study protein-protein interactions and enzyme activity.
Applications of the Structure of Peptides:
The structure of peptides has numerous applications in various fields, including medicine, biotechnology, and materials science. Peptide-based therapeutics are used to treat a wide range of diseases, including cancer, cardiovascular disease, and neurological disorders. Peptides are also used as diagnostic tools for disease detection and monitoring. In biotechnology, peptides are used as molecular probes to study protein-protein interactions and enzyme activity. In materials science, peptides are used to develop new materials with unique properties, such as self-assembly and biocompatibility.
Conclusion:
In conclusion, the structure of peptides, particularly their primary and secondary structure, is critical for their function and applications in various fields. Understanding the structure of peptides can lead to the development of new therapeutics, diagnostic tools, and research reagents. Peptides with specific structures can be optimized for their stability, activity, and specificity, and can be used to study biological processes and develop new materials with unique properties. The structure of peptides will continue to play a critical role in advancing various fields and improving human health.
